The World Health Organization declared the COVID-19 pandemic on 11 March 2020, and since then the spread of the disease has been extraordinary. The high rate of transmission and its ability to produce serious cases and mortality in certain population groups (older people and patients with previous respiratory or cardiovascular diseases) has led health and social systems to situations of extreme stress. The lack of effective treatments and vaccines has forced general individual and collective protection measures such as confinement, physical distancing, use of face masks, and reinforcement of hygiene measures.
The uncertainty regarding the future is great and very worrying in the field of health, as well as regarding the economic and social development of the entire planet, since the only effective control measure so far, physical distancing, threatens to end or modify the forms of human interaction that have been dominant until now.
In this context, the development of an effective vaccine is a social and political priority. The world is waiting for a vaccine to change this bleak picture. That is why 189 candidate products have started the complicated process of finding the vaccine against COVID-19. The race is on.
The WHO has a constantly updated list of clinical trials trying to find a vaccine against SARS-CoV-2. As of 2 October 2020, 42 candidates are being tested in humans and 151 are still in the pre-clinical phase.
Vaccine safety and assessment
To evaluate these vaccines, what are known as clinical trials are used. These are prospective, experimental studies in which the researcher «controls» the variables of the study. Clinical trials with medicines are regulated in Spain by the Royal Decree of 16 February 2004. The participating subjects are randomly assigned to the different compared interventions. This allows researchers to determine in an experimental group whether a new intervention, such as a new vaccine, is better than an older one or none at all (control group). All groups are monitored concurrently for a given period of time and the different responses obtained are compared.
Normally, subjects are randomly assigned to one group or another. Randomisation ensures that the intervention and control groups are similar in all respects except for the actual intervention, so that if differences in response are detected between the two groups, they are more likely to occur because of the studied intervention.
This type of study is considered to have the least amount of systematic errors or biases; therefore, it is the best scientific test to support evidence of the effectiveness of a particular intervention. It is considered to be the most suitable design for evaluating the efficacy of health interventions, so vaccines developed to date use this method to study their efficacy and safety.
Until the current pandemic, no coronavirus vaccine has been authorised for use in humans, because in most cases the effect of these viruses were mild and self-limiting, and therefore were not expected to have a major impact on the population. The first outbreak of SARS-CoV-1 in China prompted the development of vaccines. However, the disappearance of this virus without re-emerging in humans halted their development in the pre-clinical and early clinical stages. Vaccines against MERS-CoV, however, have remained in clinical development until today. In fact, in order to accelerate the development of new SARS-CoV-2 vaccines, the information obtained from these pre-clinical studies has been used.
The aim of developing a vaccine is to obtain a specific antibody response to the virus. The antigenic target of coronavirus vaccines is based on a surface protein (called Spike or S for short) of the virus. This protein, which is encoded by most coronaviruses, has a specific receptor-binding domain (RBD) that acts as an «entry key» by binding to the human cell receptor and fusing with the cell membrane. This virus binds to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells and then endocytes into the host cell. This step is followed by the fusion of the viral and endosomal membranes and the release of the viral genome into the cytoplasm for subsequent incorporation into the cell, which will become a factory for new viruses.
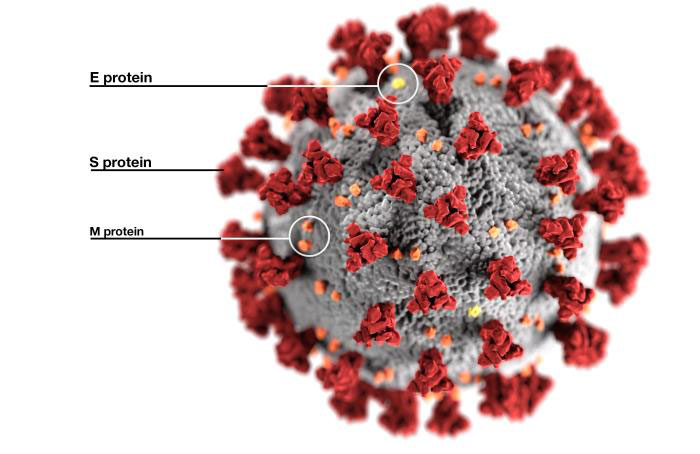
The aim of developing a vaccine is to obtain a specific antibody response to the virus. The antigenic target of coronavirus vaccines is a virus surface protein, called S in its abbreviated form. / CDC Public Health Image Library
Thus, when the virus tries to access our cells, our immune system generates antibodies as a defence response. They bind to the S-protein of the virus, especially to its receptor-binding domain (RBD), preventing the binding to the host cell and neutralising the virus. Therefore, developing antibodies that block the protein and prevent it from entering the cell is an important goal. It has now been shown that, besides acting at the time of infection, these antibodies have a certain degree of permanence protecting against re-infection.
Generally speaking, vaccine development takes a long time, often more than fifteen years. The process starts with the evaluation of vaccines in animal models, followed by a stage where pre-clinical experiments are conducted, the vaccine production process is designed, and toxicology studies are carried out. Subsequently, and after approval, Phase I clinical trials (with less than 100 people) are conducted to test the initial safety and to obtain some immunogenicity or antibody response data. If the results are satisfactory, Phase II clinical trials (involving hundreds of people) are initiated to determine the optimal immunogenicity, dose, and vaccine regimens. If the results are still adequate, Phase III trials are started again to assess efficacy and safety, but in a larger population (this time thousands of people). If the results are finally optimal, a Biologics License Application (BLA) is submitted to the relevant regulatory agencies.
The SARS-CoV-2 pandemic has accelerated the process in record time. Preliminary data from the development of the SARS-CoV-1 and MERS CoV vaccines provided pre-clinical and toxicological data for some of the vaccines in development. As a result, the first human clinical trial started already in March 2020. In addition, the pipeline of clinical phases for the clinical trial has been restructured, starting with overlapping and staggered schedules with initial phase I / II trials followed by a rapid start of phase III trials after an interim analysis of the phase I / II data. In no case has the safety assessment been cut back. In an astonishing race against time, vaccines have now reached the clinical trial stage and ten of them are in Phase III trials.
Vaccines in development against SARS-CoV-2
The development of a vaccine against SARS-CoV-2 is carried out from various approaches.
Classical approach vaccines
Including both inactivated and attenuated vaccines. The former are produced by inactivating SARS-CoV-2 using a chemical procedure. Examples of such vaccines are CoronaVac (developed by Sinovac Biotech in China), Bharat Biotech in India, and the Research Institute for Biological Safety Problems in Kazakhstan. These vaccines are administered intramuscularly and may contain adjuvants. The immune response occurs against the full virus, not just the S-protein. Clinical trials with all three Chinese candidates are in phase III.
Live attenuated vaccines are based on genetically weakening the virus so that it cannot produce the disease, but can induce immune responses similar to those produced by a natural infection. The immune response targets both structural and non-structural viral genes, with antibodies and cellular immune responses. Currently, only three live attenuated vaccines are in development, and still in the pre-clinical phase.
Viral vector vaccines
The second and more novel approach is through viral vectors and can be divided into three types: those using replication-defective vectors, those using replication-competent vectors, and those using inactive virus vectors.
In the image, a scientist from the United States Centers for Disease Control and Prevention (CDC) uses a pipette to transfer the flu virus. / CDC – Public Health Images Library
Replication-defective vector vaccines are based on the use of another type of virus that has been designed to express, in the case of COVID-19, the S protein. The virus selected as a vector cannot cause harm because it has been rendered incapable of replicating in vivo by removing parts of its genome. Most vectors are based on adenoviruses, but others are also used such as human parainfluenza or influenza viruses. They are administered intramuscularly and express the S protein to which our immune system responds by generating antibodies and a B and T cell response.
Most of these vaccines are still in the preliminary stages. The most advanced are ChAdOx1 nCoV-1947 (chimpanzee adenovirus), Janssen (adenovirus 26) – pending phase III initiation – Cansino (adenovirus 5), Gamaleya Research Institute (adenovirus 5 / adenovirus 26) – in phase III clinical trials –, and ReiThera (gorilla adenovirus) – in phase I trials.
On the other hand, vaccines based on replication-competent vectors are developed with attenuated virus strains that have been designed to express a transgene (a non-native segment of DNA), in this case the S protein. Because of their replication potential, they can generate greater induction of immunity and greater innate immune response. Currently, two vectors have begun Phase I clinical trials: a measles vaccine strain (Pasteur Institute and Themis) and an influenza-virus-based vector (Beijing Wantai Biological Pharmacy).
Finally, there are vaccines using inactivated viral vectors expressing the S protein on their surface. These vectors cannot replicate, even in immunosuppressed subjects. These technologies are currently in the preclinical stage.
Recombinant protein vaccines / VLP
The third approach is based on recombinant protein vaccines / VLPs (virus-like particles). Here we find both vaccines containing the recombinant S-protein, as well as recombinant RBD-based vaccines and VLP vaccines, expressed in insect cells, mammalian cells, yeasts, and plants.
Many recombinant protein vaccines are currently in pre-clinical development and several S-protein and RBD vaccines have already started clinical trials. Examples of these vaccines are Novavax, which has published pre-clinical and Phase I data, and Medicago’s VLP vaccine, also in clinical trials.
Nucleic acid-based vaccines
In this last section we find the DNA and RNA vaccines.
The first are based on plasmid DNA, which is very stable and contains mammalian expression promoters of the S protein. When introduced into our cells, the S gene expressed in the immunised person results in the production of S protein particles to which our immune system reacts. The disadvantage is that it requires a specific type of device to be efficiently administered. Four different DNA vaccines are currently in Phase I / II clinical trials.
RNA vaccines, similar to DNA vaccines, are also based on administering the genetic information of the antigen via lipid nanoparticles. The antigen is then expressed in our cells, triggering the immune response. They are based on mRNA or self-replicating RNA. Some RNA vaccines in development are Pfizer’s and Moderna’s, in Phase III trials, CureVac and Arcturus, in Phase I/II trials, and a candidate from Imperial College and another one from the Chinese Liberation Army is in Phase I.
To summarise, in terms of immunogenicity, the neutralising antibody response of the vaccines that have published data from their clinical trials appears to be highest for vaccines based on recombinant proteins, followed by those based on animal-derived vectors (ChAdOx) and mRNA, and lowest for inactivated or human-derived vectors (adenovirus 5). With respect to reactogenicity, inactivated vaccines and recombinant protein vaccines appear to produce few adverse effects. mRNA and vector-based (adenovirus) vaccines show a greater reaction.
A race with much remaining uncertainty
Despite unprecedented and rapid vaccine development against COVID-19, this long-distance race has only just begun. There is much to be learned in the near future.
Will the neutralising antibodies used to measure the immune response be an adequate correlate of protection? Will immunoglobulin G (IgG) responses be sufficient to generate herd immunity or will they only provide a certain degree of individual protection? What will be the duration of the protection conferred by the vaccines? Will the protective response be adequate in people with immunosenescence? How serious will be the reactogenicity of the different vaccines be? And in terms of vaccine logistics, how will the vaccines be implemented and distributed globally?
Despite all these uncertainties, for the first time in the history of humankind and pandemics, we have the possibility to develop vaccines against COVID-19 at a record speed. Let us hope that the vaccines tested in Phase III clinical trials will show the expected safety and efficacy so that they can be administered as soon as possible and succeed in controlling the spread of this pandemic.
References
Abbasi, J. (2020). COVID-19 and mRNA vaccines—First large test for a new approach. JAMA, 324(12), 1125–1127. doi: 10.1001/jama.2020.16866
Asociación Española de Pediatría. (2020). Investigación en vacunas. In Asociación Española de Pediatría, Manual de vacunas en línea de la AEP, Madrid: AEP. Retrieved from https://vacunasaep.org/documentos/manual/cap-47
Asociación Española de Pediatría (2020, 20 August). Vacunas de la COVID-19: Hasta ahora resultados predecibles, lo cual es una buena noticia. Retrieved from https://vacunasaep.org
Asociación Española de Pediatría (2020, 25 September). Vacunes del COVID-19 en estudio con datos publicados. Retrieved from https://vacunasaep.org
Callaway, E. (2020). The race for coronavirus vaccines: A graphical guide. Eight ways in which scientists hope to provide immunity to SARS-CoV-2. Nature, 580, 576–577. Retrieved from https://www.nature.com/articles/d41586-020-01221-y
Corum, J., Wee, S. L., & Zimmer, C. (2020, 26 September). Coronavirus vaccine tracker. The New York Times. Retrieved from www.nytimes.com
Folegatti, P., Ewer, K. J., Parvinder, K. A., Angus, B., Becker, S., Bellij-Rammerstorfer, S., … Pollard, A. J. (2020). Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet, 396,(10249), 467–478. doi: 10.1016/S0140-6736(20)31604-4
Krammer F. (2020, 23 September). SARS-CoV-2 vaccines in development. Nature. Retrieved from www.nature.com
Krause, P., Fleming, T. R., Longini, I., Henao-Restrepo, A. M., Peto, R., Dean, N. E., ... Henao-Restrepo, A. M. (2020). COVID-19 vaccine trials should seek worthwhile efficacy. The Lancet, 396(10253), 741–743. doi: 10.1016/S0140-6736(20)31821-3
Logunov, D. Y., Dolzhikova, I. V., Zubkova, O. V., Tukhvatullin, A. I., Shcheblyakov, D. V., Dzharullaeva, A. S., ... Gintsburg, A. L. (2020). Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. The Lancet, 396(10255), 887–897. doi: 10.1016/S0140-6736(20)31866-3
Mulligan, M. J., Kirsten, E. L., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., ... Jansen, K. U. (2020). Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. doi: 10.1038/s41586-020-2639
Nature Reviews Microbiology. (2011). Microbiology by numbers. Nature Reviews Microbioly, 9, 628. doi: 10.1038/nrmicro2644
Sahin, U., Muik, A., Derhovanessian, E., Vogler, I., Kranz, L. M., Vormehr, M., ... Tuereci, O. (2020). Concurrent human antibody and TH1 type T-cell responses elicited by a COVID-19 RNA vaccine. MedRxiv. doi: 10.1101/2020.07.17.20140533
Tung, T. L., Andreadakis, Z., Kumar, A., Gómez Román, R., Tollefsen, S., Saville, M., & Mayhew, S. (2020). The COVID-19 vaccine development landscape. Nature Reviews, 19, 305–306. Retrieved from Nature Reviews.
Wikipedia. (2o2o). Vacuna. Retrieved from es.wikipedia.org
World Health Organization. (2020). Alerta y respuesta mundiales (GAR). Retrieved from www.who.int
World Health Organization. (2020). WHO vaccine pipeline tracker. Retrieved from www.who.int
World Health Organization. (2020, 2 October). Draft landscape of COVID-19 candidate vaccines. Retrieved from www.who.int
Xia, S., Duan, K., Zhang, Y., Zhao, D., Zhang, H., Xie, Z., ... Yang, X. (2020). Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes. Interim analysis of 2 randomized clinical trials. JAMA, 324(10), 951–960. doi: 10.1001/jama.2020.15543
Zhu, F. C., Guan, X. H., Li, Y. H., Huang, J. Y., Jiang, T., Hou, L. H., ... Chen, W. (2020) Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet, 396(10249), 479–488. doi: 10.1016/S0140-6736(20)31605-6
Zhu, F. C., Li, Y. H., Guan, X. H., Hou, L. H., Wang, W. J., Li, J. H., ... Chen, W. (2020). Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet, 395(10240), 1845–1854. doi: 10.1016/S0140-6736(20)31208-3