Toxic microalgae and global change
Why have proliferations increased along the Mediterranean coast?
The ocean and the continent converge in a very narrow line that is, nonetheless, truly relevant to the health, leisure, and economy of our society. The Mediterranean coastline has undergone major changes over the last fifty years, which is evident in the alteration of its microalgae species. The proliferation of dinoflagellates is now common in microscopic organism communities in this ecosystem as a result of the modifications caused by humans and climate change. The increased frequency with which toxic microalgae blooms are detected has been key to raising awareness of this change.
Keywords: phytoplankton, microalgae, marine toxins, Ostreopsis.
Marine microalgae and mobility in a globalised world
In a globalised and highly communicated planet like the one we live on, human movement from one end of the world to the other takes a matter of a few hours. Goods usually take a little longer, but they can also end up in places far away from their shipping origin in only a matter of days, weeks, or months. As part of this coming and going of people and products, we often also accidentally and involuntarily transport some living organisms, both those visible to the naked eye and others that are microscopic (Hallegraeff, 1998). Here we will focus on showing how the geographical distribution of marine microorganisms, specifically microalgae, is changing – often by expanding towards higher latitudes – and the relationship this has with global warming and human activities.
«The human impact translates into increases in the quantity and quality of the nutrients that are dumped into rivers and the sea»
The problem becomes more apparent when we understand that some of these species produce toxins that can affect human health or marine ecosystems. In the terrestrial ecosystem, the COVID-19 pandemic we are currently experiencing is a good example of the rapid spread of a pathogen from a distant source to the myriad places it has come to affect. This redistribution of organisms is a common occurrence on a globalised planet. However, the problem only becomes evident with the spread of species that negatively impact human health, ecosystems, or the economy.
Returning to aquatic ecosystems, microalgae are the main primary producers in the ocean. The best-known examples are phytoplankton which, as their name suggests,1 are a group of photosynthetic organisms with insufficient capacity for movement to exceed the physical energy of the sea and are, therefore, carried by marine currents and waves. Phytobenthic organisms can be found covering the sea floor, or more generally, between, on, or near grains of sand.2 In recent decades, marine microalgae, whether planktonic or benthic, are experiencing a geographical species redistribution. Here, we will focus on the case of the Mediterranean, a semi-enclosed sea surrounded by densely populated land that is generally considered to be oligotrophic and with low tidal forces.
Human stresses on the coastline and proliferations of dinoflagellates
Seasonal phytoplankton dynamics in temperate latitudes have their optimal periods in late winter and early spring when microalgae proliferate and achieve high abundances. With agitation of the water column in winter, nutrients ascend from the deep waters to the surface layers and, coinciding with the increase in light and temperature, causes the growth of microalgae communities, mainly diatoms. During the spring, different planktonic communities follow one another and consume the nutrients of the surface layers until they are almost exhausted. The sun heats the surface of the sea which forms the thermocline – a density structure that separates the superficial layers from deeper ones; this physical barrier does not allow the arrival of more nutrient supply from the bottom. Therefore, during the summer, the abundance of planktonic microalgae in superficial Mediterranean waters is low, which makes them clear and transparent. Another, more moderate, production peak occurs in autumn, when high levels of irradiance again coincides with an increase in nutrients, caused by rupture of the thermocline and mixing of the water columns.

Figure 1A. Examples of coastal transformations as a product of human action: The Empuriabrava urbanisation project (Castelló d’Empúries, Girona, Spain), built on the seafront in a wetland area. Coastal wetlands have been virtually wiped out from the Mediterranean coastal ecosystem. / Photo: Jordi Camp
Humans exert a high pressure on the coast and, in the Mediterranean, a semi-enclosed sea surrounded by such a high population, this effect is even more noticeable. During the summer months, tourism causes the populations of some coastal towns to multiply by ten to a hundred-fold. This human impact translates into increases in the quantity and changes in the quality of the nutrients that are dumped into rivers and the sea, which cannot always be completely eliminated by sewage treatment systems. Thus, coastal waters become richer in nutrients as a result of human pressure exerted from the continental. Photosynthetic organisms (primary producers) take advantage of these nutrients of anthropic origin to grow.
Furthermore, urbanisation of the coastal zone has rendered the soil waterproof (Figure 1A); wetland areas have been virtually obliterated – paved over – from the coastal ecosystem, and what used to act in past decades as a natural filter for the nutrient-rich waters provided by the continent are now paved streets and waterways that do not filter but rather, dump everything that reaches them into coastal waters (Camp et al., 1998). Thus, they are mere conduits of enriched water which make the seawater on the immediate coast rich in nutrients, which primary producers use to grow.
«By confining coastal marine waters in ports, humans have created optimal conditions for the growth of these “red tide”-forming microalgae»
Coastal foundations and rigidity also have an important effect on the sea. On the one hand, the construction of dams in riverbeds has significantly reduced the steady supply of sediments to coastal areas. In the past, these sediments were transported by coastal currents and distributed along the coast so that they provided sand to the beaches. The rhythm of nature has also been disrupted on urban beaches, with dune systems and coastal lagoons being replaced by promenades and beachfront buildings. The winter storms that carry away the sand from the beaches leave eroded beaches which cannot be restored with sand from the dune system (because none is present), or by river sediments (because these have been greatly reduced). Part of the sand washed away by the sea accumulates in breakwaters built perpendicularly to the coastline, which cut off water circulation.
Therefore, in many cases, a huge amount of sand must be moved in order to recover beaches that would have otherwise recovered on their own in the past. Thus, year after year, governments allocate part of their budgets to carry out various beach regeneration projects: costly interventions – both from an economic and an ecological point of view – which are ephemeral because they only last until the next storm arrives. Moreover, the forecast in a climate change scenario is that such storms will become more frequent and intense, such as storm Gloria reminded us at the beginning of 2020.

Figure 1B. Harbour and beach in Cambrils, Tarragona (Spain). Building harbours and breakwaters increases the volume of confined waters, an ideal habitat for dinoflagellate blooms./ Photo: Jordi Camp
Seaports and breakwaters (Figure 1B), however, play another role. By confining waters in order to provide shelter for boats, bodies of water with high residence times that allow for the growth and accumulation of microalgae are created. Calm, shallow, nutrient-rich waters with long residence times are ideal conditions for the growth of dinoflagellates, a group of phytoplankton that produce the proliferations and colour changes popularly known as red tides and scientifically referred to as harmful algal blooms. Moreover, within the phytoplankton groups, dinoflagellates have the highest number of harmful or toxic species.
«Thirty years ago, Ostreopsis was a rare genus in the Mediterranean, detected very sporadically and in limited abundance»
According to the mandala published by Ramon Margalef (Margalef, 1978; Margalef et al., 1979; Figure 2), the dinoflagellates that cause red tides proliferate because of the combination of high levels of nutrients with calm waters. As explained above, nutrient inputs are often related to agitation of the water column because it brings nutrients from the bottom to the surface; therefore, it is unusual to find elevated nutrient levels associated to still water; these circumstances occur naturally in bays or near river mouths. By confining coastal marine waters in ports, humans have created optimal conditions for the growth of these red tide-forming microalgae. When monitoring programmes for toxic species were implemented in the Mediterranean in the 1990s, bloom-forming dinoflagellates were very well represented. This surprised experts because, according to Margalef, the Mediterranean did not have the appropriate characteristics for red tides to take place. Our hypothesis (Vila et al., 2001) was that the recreational use of coastal waters favours dinoflagellate proliferations. The construction of ports – there are currently about fifty along the Catalan coast, i.e., the equivalent of one every 8–10 km along the coastline – generates semi-enclosed water bodies with notable concentrations of nutrients, high water residence times (about twenty days), low turbulence, and low advection compared to unconfined waters, all of which favours these blooms.
Both dinoflagellate behavioural strategies (swimming, active vertical migration, and aggregation) and production of toxic compounds, are involved in reducing zooplankton predation (see Selander et al., 2015; Smayda, 1997). In addition, the concentrations of inorganic nutrients and their stoichiometric ratios indicate that areas characterised by high population densities have higher levels of ammonium and phosphates and a more limited silicate content. Therefore, they favour the growth of dinoflagellates in relation to diatoms because the latter require silicate to build their cellular covers (frustules).
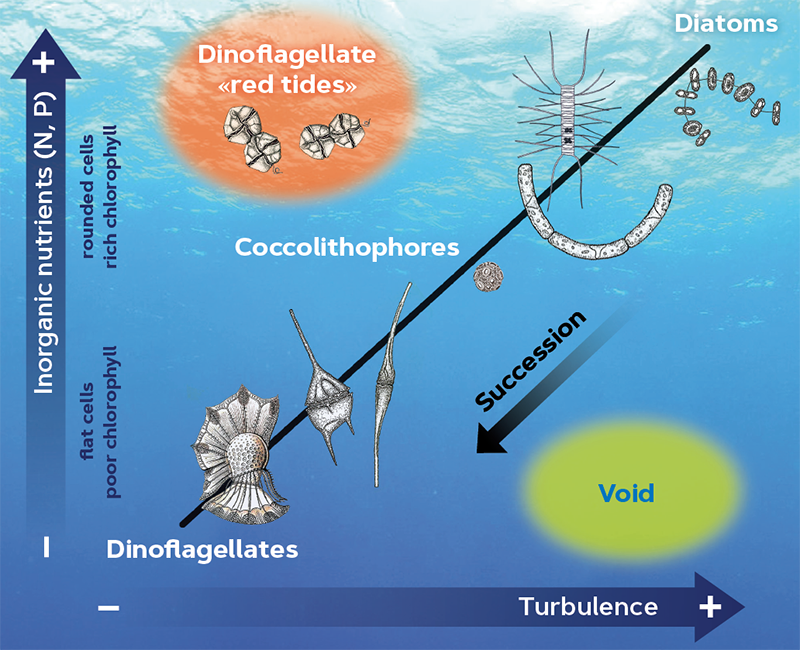
Figure 2. Ramon Margalef’s mandala is a schematic representation showing how the seasonal succession of the main phytoplankton groups depends on nutrient concentration and water turbulence or agitation. / Source: visual adaptation of Margalef’s mandala (1978).
Finally, ports are ideal environments in which organisms with some form of resistance (cysts or seeds) can remain confined in the sediment until new environmental conditions induce them to germinate (Anderson & Wall, 1978). Dinoflagellate blooms in harbours occur because the active growth of a small part of the germinating population is amplified by swimming and aggregation, reduction of zooplankton predation, and favourable physical factors indicated above. Therefore, a coast with many ports facilitates the colonisation and the establishment of new, non-native species. This has occurred in the case of several species from the genus Alexandrium (Vila et al., 2001) which can generate toxins (saxitoxins) that produce paralysing symptoms in people who eat bivalve molluscs contaminated with these organisms (Berdalet et al., 2016).
Beaches covered in microalgal mats
Some 20 or 25 years ago, blooms of benthic dinoflagellates of the genus Ostreopsis began to be detected during the summer months on different Mediterranean beaches. At that time, this genus was known in tropical areas to form part of the group of microorganisms (microbiota) accompanying a toxic dinoflagellate of the genus Gambierdiscus, which caused a tropical food poisoning known as ciguatera (Friedman et al., 2017). The increase in sea water temperature seems to have been the trigger for the establishment of several tropical species in the Mediterranean, which in some cases, have replaced native communities. This is known as the tropicalisation of the Mediterranean (Bianchi et al., 2018).

Figure 3 (left to right). Until a few years ago, it was not common to find benthic dinoflagellates of the genus Ostreopsis in the waters of the Mediterranean; this species is more typical in tropical areas. The increase in sea temperature appears to have caused these species to settle a new location. The pictures show: A) water colouration in a shallow beach covered by a proliferation of the benthic dinoflagellate Ostreopsis / Photo: Elisa Berdalet. B) State of a shallow seabed covered in an Ostreopsis bloom / Photo: Magda Vila.
Ostreopsis secretes a mucous and sticky substance that keeps it softly anchored to macroalgae (Figure 3). This ability allows it to stay on beaches, close to the surface, and to proliferate relatively quickly, forming a dense carpet of microalgae and mucilage that covers the sea floor. In response to wave agitation or other factors, Ostreopsis detaches itself from the macro-algae and can be found swimming in the water column or floating on the surface, forming what in France is known as water flowers. These proliferations have been associated with massive mortalities of marine fauna that have little or no mobility (e.g., sea urchins, mussels, etc.), perhaps because of the limited oxygen availability associated with the extensive mucilaginous layer covering the seabed, or because of the production of certain toxic substances (Giussani et al., 2016; Shears & Ross, 2009).
Indeed, Ostreopsis produces ovatoxins, which are analogous to palytoxin. Palytoxin has been associated with lethal cases of food poisoning in the Indian Ocean in people who had consumed seafood contaminated with these compounds. These toxins enter the food chain when fauna feed on Ostreopsis-coated macroalgae, transmitting these toxins up to higher trophic levels, including to humans (Berdalet et al., 2017). In the Mediterranean, certain toxins associated with Ostreopsis have been detected in diverse marine fauna; however, food poisoning does not appear to be a problem at this time. Instead, massive proliferations of Ostreopsis in this area have been associated with mild respiratory irritations (rhinorrhoea, fever, general discomfort, and eye and nose irritation, etc.) in bathers and people exposed to sea spray on several beaches in Algeria, Spain, France, Italy, and Greece (Vila et al., 2016).
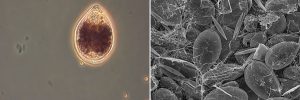
Figura 3 (left to right). C) Ostreopsis cell through an optical microscope / Photo: Magda Vila. D) the appearance of a benthic proliferation dominated by Ostreopsis under a Scanning Electron Microscope (SEM): the network of filaments secreted by the cells can be seen. / Photo: Magda Vila i José Manuel Fortuñó.
A similar aerosol exposure mechanism has been confirmed as the cause of respiratory irritation symptoms during the blooms of Karenia brevis in the Gulf of Mexico (Fleming et al., 2011). In this case, the enormous scientific investment made by several institutions over decades of studies has provided sound knowledge that has brought about the adequate management of ecological and health risks in this area. However, in the Mediterranean, the efforts made to link these symptoms with the presence of toxins in the aerosol have provided little empirical demonstration so far (Ciminiello et al., 2014). It has been hypothesised that the irritation was not caused by the toxins themselves, but rather, by some other cell component or fragment that might have triggered some kind of reaction. It has even been speculated that the problem may have been caused by some of the microorganisms (bacteria or virus) associated with Ostreopsis (Bellés-Garulera et al., 2016; Casabianca et al., 2013). However, bathers with skin wounds that suffered skin irritations, were also successfully treated with topical antibiotics. All this suggests that more than one factor contributes to the various undesirable effects of Ostreopsis proliferations.
Final considerations
It is evident that the Mediterranean coast has changed a lot over the last fifty years. The natural habitat of beaches and cliffs has been replaced by an artificial one comprising harbours and breakwaters, which have enclosed not only boats but also water and microalgae. Moreover, a habitat change also involves a change in species. Wetlands have been reduced to a minimum and shoreline developments have multiplied, significantly changing the flow of sediments and nutrients into the sea. Finally, with global warming, the temperature of the sea has increased, and some invasive species have arrived and settled these areas. Thus, the communities of microalgae species that existed fifty years ago have been modified or «enriched» leading, in some cases, to blooms of toxic dinoflagellates.
«The ocean and the continent converge in a very narrow line that is nonetheless truly relevant to the health, leisure, and economy of our society»
Planktonic proliferations of the genus Alexandrium are now frequent in the Mediterranean, and since the 1990s, they have been monitored on a weekly basis with by programmes that guarantee the safety of the food products that arrive at the fish markets and to fishmongers. Monitoring benthic microalgae is progressing more slowly because of a great lack of knowledge about Ostreopsis species until recently. Thirty years ago, this organism was a rare genus in the Mediterranean, which was detected very sporadically and in limited abundance. Now it has become a public health and environmental problem, which every year mobilises scientists and administrations, and concerns the residents of the beaches affected by these massive proliferations. By studying countries for which we currently have more information about these blooms (Mangialajo et al., 2011), it appears that what began as a massive proliferation on a particular beach during the first few years has become an expansion with multiple focal points which affects many beaches, first in Italy and France and, in the last five years, also in Catalonia. There are still many gaps in our understanding of Ostreopsis toxicity. However, knowledge of how it proliferates and coordination between scientists and environmental and health policy-makers has made it possible to manage the phenomenon properly and to minimise ecological and health risks.
Over the last fifty years, we have transformed our waters through direct coastal interventions such as the construction of ports and breakwaters. However, we have also constructed promenades and housing developments along the coast, with new river channelling and dams. As a result of human activity, atmospheric CO2 and the global temperature of the planet have also increased. These direct or indirect human actions could plausibly explain why microalgae proliferation on Mediterranean coasts has increased in recent decades.
The ocean and the continent converge in a very narrow line that is nonetheless truly relevant to the health, leisure, and economy of our society. Maintaining our shores in a good ecological state can only be achieved if the activity carried out inland also upholds the standards of sustainability. In order to keep our coastline in good condition, we must rethink the world we want to live in and act accordingly.
Notes
- Plankton comes from the Greek word πλαγκτός and means «wanderer». (Go back)
- Benthos, βένθος, means «depth of the sea». (Go back)
References
Anderson, D. M., & Wall, D. (1978). Potential importance of benthic cysts of Gonyaulax tamarensis and G. excavata in initiating toxic dinoflagellate blooms. Journal of Phycology, 14(2), 224–234. https://doi.org/10.1111/j.1529-8817.1978.tb02452.x
Bellés-Garulera, J., Vila, M., Borrull, E., Riobó, P., Franco, J. M., & Sala, M. M. (2016). Variability of planktonic and epiphytic vibrios in a coastal environment affected by Ostreopsis blooms. Scientia Marina, 80(S1), 97–106. https://doi.org/10.3989/scimar.04405.01A
Berdalet, E., Fleming, L. E., Gowen, R., Davidson, K., Hess, P., Backer, L. C., ... Enevoldsen, H. (2016). Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. Journal of the Marine Biological Association of the United Kingdom, 96(1), 61–91. https://doi.org/10.1017/S0025315415001733
Berdalet, E., Tester, P. A., Chinain, M., Fraga, S., Lemée, R., Litaker, W., ... Zingone, A. (2017). Harmful algal blooms in benthic systems: Recent progresses and future research. GEOHAB Oceanography (special issue), 30(1), 36–45. https://doi.org/10.5670/oceanog.2017.108
Bianchi, C. N., Caroli, F., Guidetti, P., & Morri, C. (2018). Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. Journal of the Marine Biological Association of the United Kingdom, 98(1), 1–12. https://doi.org/10.1017/S0025315417000819
Camp, J., Masó, M., Vila, M., Delgado, M., Garcés, E., & Torres, M. (1998). Características ambientales del litoral Mediterráneo Noroccidental; situación actual e implicaciones: El caso de Cataluña. In Actas de la V Reunión Ibérica de Fitoplancton Tóxico. ANFACO-CECOPESCA.
Casabianca, S., Casabianca, A., Riobó, P., Franco, J. M., Vila, M., & Penna, A. (2013). Quantification of the toxic dinoflagellate Ostreopsis spp. by qPCR assay in marine aerosol. Environmental Science & Technology, 47(8), 3788−3795. https://doi.org/10.1021/es305018s
Ciminiello, P., Dell’Aversano, C., Iacovo, E. D., Fattorusso, E., Forino, M., Tartaglione, L., Benedettini, G., Onorari, M., Serena, F., Battocchi, C., Casabianca, S., & Penna, A. (2014). First finding of Ostreopsis cf. ovata toxins in marine aerosols. Environmental Science & Technology, 48(6), 3532–3540. https://doi.org/10.1021/es405617d
Fleming, L. E., Kirkpatrick, B., Backer, L. C., Walsh, C. J., Nierenberg, K., Clark, J., ... Baden, D. G. (2011). Review of Florida red tide and human health effects. Harmful Algae, 10(2), 224–233. https://doi.org/10.1016/j.hal.2010.08.006
Friedman, M. A., Fernandez, M., Backer, L. C., Dickey, R., Bernstein, J., Schrank, K., ... Fleming, L. E. (2017). An updated review of ciguatera fish poisoning: Clinical, epidemiological, environmental, and public health management. Marine Drugs, 15(3), 72. https://doi.org/10.3390/md15030072
Giussani, V., Costa, E., Pecorino, D., Berdalet, E., De Giampaulis, G., Gentile, M., ... Faimali, M. (2016). Effects of the harmful dinoflagellate Ostreopsis cf. ovata on different life cycle stages of the common moon jellyfish Aurelia sp. Harmful Algae, 57, 49–58. https://doi.org/10.1016/j.hal.2016.05.005
Hallegraeff, G. M. (1998). Transport of toxic dinoflagellates via ships’ ballast water: Bioeconomic risk assessment and efficacy of possible ballast water management strategies. Marine Ecology Progress Series, 168, 297–309. https://doi.org/10.3354/MEPS168297
Mangialajo, L., Ganzin, N., Accoroni, S., Asnaghi, V., Blanfuné, A., Cabrini, M., ... Lemée, R. (2011). Trends in Ostreopsis proliferation along the Northern Mediterranean coasts. Toxicon, 57(3), 408–420. https://doi.org/10.1016/j.toxicon.2010.11.019
Margalef, R. (1978). Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanologica Acta, 1(4), 493–509.
Margalef, R., Estrada, M., & Blasco, D. (1979). Functional morphology of organisms involved in red tides, as adapted to decaying turbulence. In D. L. Taylor, & H. H. Seliger (Eds.), Toxic dinoflagellate blooms (pp. 89–94). Elsevier.
Selander, E., Kubanek, J., Hamberg, M., Andersson, M. X., Cervin, G., & Pavia, H. (2015). Predator lipids induce paralytic shellfish toxins in bloom-forming algae. Proceedings of the National Academy of Sciences of the United States of America, 112(20), 6395–6400. https://doi.org/10.1073/pnas.1420154112
Shears, N. T., & Ross, P. M. (2009). Blooms of benthic dinoflagellates of the genus Ostreopsis: An increasing and ecologically important phenomenon on temperate reefs in New Zealand and worldwide. Harmful Algae, 8(6), 916–925. https://doi.org/10.1016/j.hal.2009.05.003
Smayda, T. J. (1997). Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnology and Oceanography, 42(5, Part 2), 1137–1153. https://doi.org/10.4319/lo.1997.42.5_part_
2.1137
Vila, M., Abós-Herràndiz, R., Isern-Fontanet, J., Àlvarez, J., & Berdalet, E. (2016). Establishing the link between Ostreopsis cf. ovata blooms and human health impacts using ecology and epidemiology. Scientia Marina, 80(S1), 107–115. https://doi.org/10.3989/scimar.04395.08A
Vila, M., Garcés, E., Masó, M., & Camp, J. (2001). Is the distribution of the toxic dinoflagellate Alexandrium catenella expanding along the NW Mediterranean coast? Marine Ecology Progress Series, 222, 73–83. https://doi.org/10.3354/meps222073