Medications against drugs
Development of medications to prevent and treat substance use disorders

Substance use disorder (SUD) is a significant public health concern. Unfortunately, there are few safe and effective medications to treat SUD and efficacy is suboptimal. There are important financial and scientific obstacles to develop new compounds, but recent advances in the discovery of new brain receptors and neurocircuits are offering opportunities to develop new pharmacotherapies. A systematic scientific approach to develop medications is required to demonstrate their safety and efficacy, bring it to market, and prescribe it to patients. The purpose of this manuscript is to provide a general overview of the challenges and opportunities in medications development for SUD, describe the phased approach of this development, the medications approved, and those that appear most promising.
Keywords: medications development, substance use disorders, treatment, clinical trials.
Introduction
Medications development to prevent and treat illicit substance use disorder (SUD) is a high public health priority that requires scientific and financial collaborations among academic investigators, government agencies, and pharmaceutical industry. According to the National Survey of Drug Use and Health of the United States (NSDUH) of 2019, there were approximately 8.3 million individuals with SUD but only 1.5 million were treated with a medication approved by the Food and Drug Administration (FDA) for the disorder (SAMHSA, 2019). Thus, most patients with SUD do not receive a medication to treat it. This is, in part, because of a lack of access to pharmacological treatment, but mainly due to the dearth of medications approved for their disorder and their limited efficacy. This treatment gap needs to be urgently addressed with more pharmacotherapies that are safe, effective, and available to patients with SUDs (Rasmussen et al., 2019).
Despite the critical need, there is only a handful of biotechnology or pharmaceutical companies interested in developing medication for SUD. This is due in part to the misconception that there is low return on the investment and the challenges posed by the target patient population, due to their multiple medical and psychiatric comorbidities, unpredictable motivation to stay in treatment, and poor treatment outcomes. However, the current market of medications approved for opioid use disorders exceeds $1.2 billion per year and multiple strategies to incentivize the pharmaceutical industry to get into the SUD field have been proposed, including vouchers and lengthening the duration of drug patents, but they have not been implemented and more companies are abandoning the development of psychotherapeutics for brain disorders, including SUD (Skolnick & Volkow, 2012).
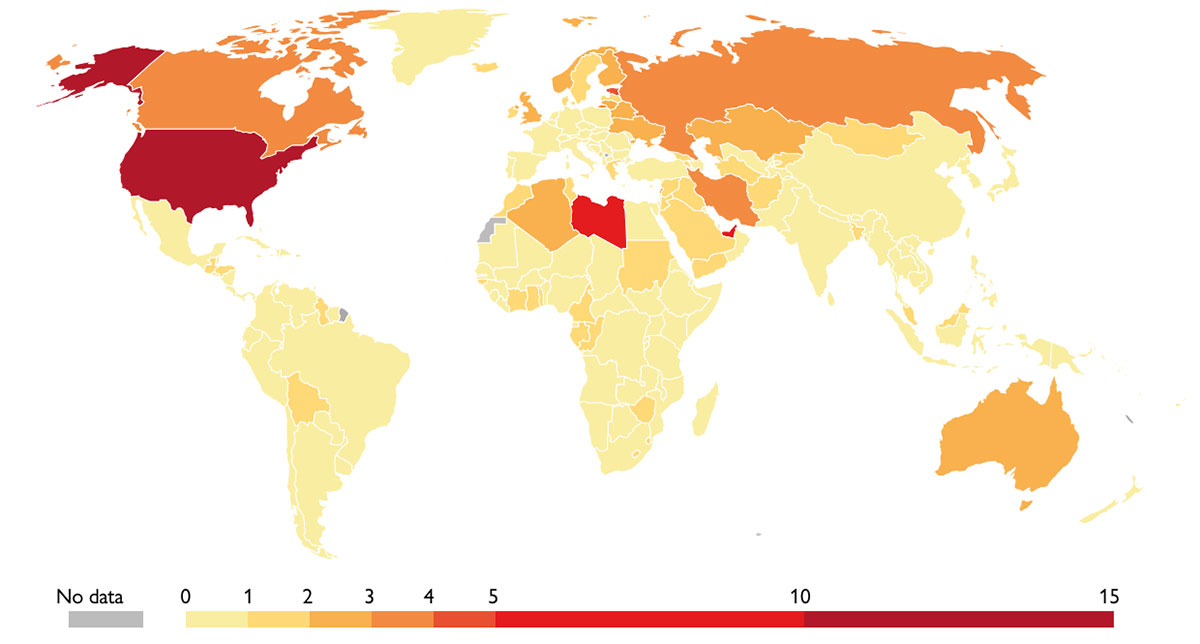
Map showing the world-wide death rates from opioid overdoses for 2017, measured as the number of deaths per 100,000 individuals. With 13.34 death per 100,000 people, the United States lead the raking above countries such as Libya (7.27) and the United Arab Emirates (5.4). / IHME. Global Burden of Disease (GBD) / Our World in Data
Challenges and opportunities
The development of SUD medications requires significant financial and scientific support. The average time from the discovery of a new compound to obtain approval from the regulatory agencies, for example the FDA, is about 14 years, if everything goes well. The approximate cost of a successful medication development from discovery to approval is about $2.4 billion. On the other hand, investing in developing safe and effective medications to treat SUDs can save millions of dollars in loss of productivity and, more importantly, many lives. Therefore, increasing the treatment options for SUD patients is clearly cost-effective as well as profitable.
From the scientific point of view, SUD is a chronic clinical condition characterized by the compulsive use of a drug, despite the physical, psychological, and social negative consequences of its use. The initiation and progression of drug use is associated with biological, social, and psychological risk factors. Chronic drug use has been associated with brain changes that may explain the changes in life priorities and clinical manifestations such as drug withdrawal syndrome and craving, which perpetuate the drug use.
«Most patients with substance use disorder do not receive a medication to treat it»
Scientific advances in understanding the effects of acute and chronic use of drugs on the brain and its neurotransmitters and neurocircuits are offering unprecedented opportunities to discover new pharmacological targets and the development of new medications to treat SUDs. Furthermore, advances in understanding the genetic and epigenetic basis of SUD have opened new opportunities to learn about pharmacogenetics of the individual effects of drugs of abuse as well as the safety and efficacy of medications that are allowing more individualized pharmacological approaches.
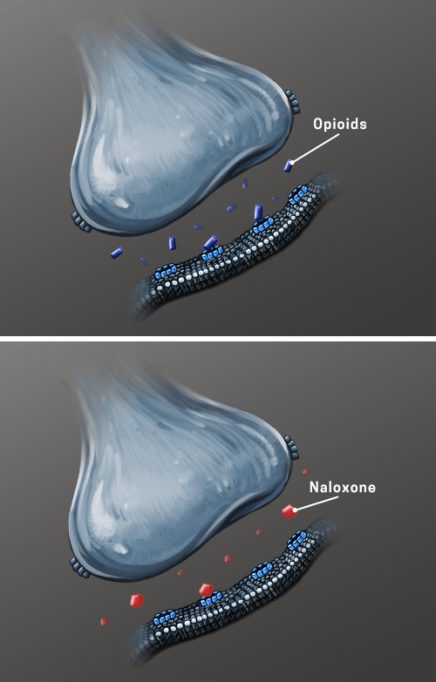
Advances in immunology are also making possible the development of biologics such as vaccines, monoclonal antibodies, and enzymes, which can alter the pharmacokinetic profile of drugs and be used for the treatment of SUDs as well as prevention of drug overdoses. Anti-drug vaccines produce an immunological response characterized by the production of antibodies against the specific drug of abuse. Monoclonal antibodies produced in the laboratory bind to the drug of abuse and create a large antigen-antibody which does not cross the blood-vein barrier and thus prevents the access of the drug to the brain. The ultimate effect of vaccines and monoclonal antibodies is to produce a pharmacokinetic antagonism and protect the central nervous system from the effects of the drug of abuse and its neurobehavioral consequences. Engineered enzymes that are being developed to treat SUD have the property of significantly accelerating the catabolism of the drug of abuse at a pace that is much faster than the natural enzymes. That way, when the drug of abuse enters the blood system, the engineered enzyme will break down the drug in plasma before it is able to enter the brain and, thus, prevent the neurobehavioral effects of the drug, including the brain reward mechanisms responsible for the compulsive use (Montoya, 2016).
Clinical research for new medications to treat substance use disorder entails the risk that the study medication may have addictive properties which could add another addiction to the patient. Usually, Phase I of clinical research counts on the collaboration of volunteers who are not seeking treatment. On the image, a clinician of the National Institute of Drug Abuse examines an ambulatory patient going through a research treatment. / NIDA (NIH)
Medications development phases
In order to have new medications approved by regulatory agencies and reach patients, new compounds must go through a rigorous process of scientific and unbiased evaluation, which includes comprehensive pre-clinical and clinical research. For SUD, this process has some unique aspects, given that the disorder is associated with the compulsory intake of an illegal substance, which may interact pharmacologically with the study medication. Besides, there is also the risk that the study medication may have addictive properties and increase the risk of adding another addiction to the patient.
Preclinical phase
In the preclinical research phase, the compound is tested in animals to determine its potential safety to be administered to humans and its preliminary efficacy in the pertinent animal models of SUD. Animal studies are critical in this development process. For SUD, it is necessary to evaluate the abuse liability of new compounds and determine the risk of developing addiction. A compound that demonstrates abuse liability in animals is unlikely to be approved to be tested in humans for further development. Drug discrimination, conditioned place preference, and drug self-administration paradigms help to determine if animals can recognize or prefer the medication over food or other reinforcers. Animal studies are also important to determine potential toxicological effects and adverse interactions with other drugs of abuse or other medications. One of the concerns about animal models of SUD is the heterogeneity of SUDs and the predictability of such models to the human condition. However, they are widely used, and investigators need to continue using them until they can be validated with medications that have demonstrated efficacy (Banks et al., 2019).
Clinical phase
The clinical research phase is divided in four phases. They are described by the FDA as Phase I to Phase IV. Phase I studies are also called «first-in-human» studies because the goal is to determine the medical safety of administering the new compound to humans. They usually include a relatively small number of study participants who may or may not have a history of drug use and who are financially reimbursed to be exposed to the potential risks of the new medication. This type of study may be followed by a second Phase I study, usually called «Phase Ib», to determine the pharmacological interactions of the new medication and drugs of abuse. This type of study is particularly relevant when it is suspected that the study medication may increase the risk or exacerbate the effects of drugs of abuse. An important component in the Phase I studies is the evaluation of the abuse liability of the new medication. These studies are conducted in human research volunteers who have experience with the effects of the other drugs in the same pharmacological class. For ethical reasons, these studies are conducted in individuals who are not seeking treatment, given that they will be exposed to drugs which may have abuse potential. The standard measures include subjective ratings of drug effects including drug «liking», euphoria, somnolence, and other cognitive and behavioral effects (Epstein et al., 2006).
Phase II clinical trials are often called «proof-of-concept» studies because the purpose is to determine the preliminary efficacy of the medication in patients who are seeking treatment. These studies are usually conducted in outpatient settings and with samples that can range from 50 to 100 study participants. In consequence, it is critical to select endpoints or outcome measures that represent a clinically meaningful improvement of the SUD that is being evaluated. The outcome measure is going to depend on the specific SUD of the patient. Toxicology tests in urine and other human fluids allow to somewhat objectively assess the frequency and intensity of drug use and the severity of the SUD. However, clinically, a drug test result is not an indicator of the whole functioning of the individual. Therefore, other outcome measures have been developed and validated with the goal of obtaining a more comprehensive idea of the clinical situation of the patient. They include, for example, the Clinical Global Impression (CGI), or the Addiction Severity Index (ASI) (Kiluk et al., 2016; Kleykamp et al., 2019; Loflin et al., 2020; Montoya et al., 1995).
Phase III clinical trials are the gold standard to establish the safety and efficacy of a compound. They are usually conducted in large sample of patients who are expected to resemble the «real world» patients with the disorder. Their aim is to confirm the efficacy demonstrated in the Phase II trials and serve as basis to support the specific treatment indication of the medication. Therefore, the proper selection of the study endpoints and statistical approach in the previous phase are essential because study results are presented to regulatory agencies with the goal of obtaining approval to market the product (Yu et al., 2008).
Phase IV studies are usually referred as post-marketing surveillance and usually carried out by the sponsor pharmaceutical company. The purpose is to identify rare adverse effects of the medication that were not discovered in the previous phases. This phase also includes studies in populations that were included in the studies in the previous phases.
Medications approved and under development
Currently, there are some medications for SUDs that have gone through this rigorous evaluation and approval process. For opioid use disorder, methadone was approved by the FDA in 1972 and it is widely used in most of countries. Buprenorphine alone and in combination with naloxone were approved in 2002 and has quickly gain acceptance among prescribers because of its unique pharmacological property of being an opioid partial agonist. More recently, in 2016, a six-month implant and, in 2017, a long-acting injectable formulation of buprenorphine were approved.
For relapse prevention of opioid use disorder, oral naltrexone (an opioid antagonist) has been approved since 1984 but adherence to the prescription of this medication is very poor. To overcome this barrier, a long-acting (monthly) formulation of naltrexone was approved in 2010. Its acceptance by clinicians and patients has been low because patients must be detoxified and not using opioids for at least two weeks before the first injection, to prevent precipitating an opioid withdrawal (Coffa & Snyder, 2019).
According to the NSDUH, in 2019 in the U.S., there were 746,866 patients in treatment with buprenorphine, 637,157 with methadone, and 77,872 with naltrexone (SAMHSA, 2019). Other medications that are part of the armamentarium of treatments but no included in the NSDUH are naloxone and lofexidine. Naloxone was approved in 1971 for the treatment of opioid overdose and more recently, given the opioid use epidemic in the U.S., an intranasal formulation was developed and was approved by the FDA in 2015. This formulation has saved the life of many people who have overdosed with opioids. However, the overdose epidemic in the United States persists and in 2018 there were almost 50,000 opioid overdose deaths, with nearly 35,000 of them due to fentanyl or other synthetic opioids besides methadone (Kreek et al., 2019). Lofexidine is an anti-hypertension medication that was approved by the FDA in 2018 to treat the symptoms of opioid withdrawal in opioid-dependent patients who discontinue the use of opioids. This medication can help patients who want to be detoxified from opioids and possibly transition to relapse prevention with naltrexone (Yu et al., 2008).
To respond to the public health need of having more medications available to treat SUDs, in 1989, the U.S. Congress mandated the creation of the Medications Development Program at the National Institute on Drug Abuse (NIDA) with the goal of rapidly and efficiently respond to the drug use crisis by supporting and funding the development of medications for SUDs. Since its inception, the program has evaluated hundreds of medications and only a few have reached the finishing line of obtaining FDA approval. The program has been credited for supporting the development of naltrexone, levo-alpha-acetylmethadol (LAAM), buprenorphine alone and in combination with naloxone, buprenorphine implant, and lofexidine (Johnson & Vocci, 1993; Vocci & Ling, 2005).
After more than 30 years of research, there are no FDA-approved medications to treat patients with cannabis, cocaine or methamphetamine use disorder. This is particularly unfortunate because the use disorder and overdose deaths with these drugs have dramatically increased in recent years. It has been reported that between 2010 and 2018, the overdose deaths for cocaine and methamphetamine have more than quadrupled reaching a total of 14,666 and 12,676, respectively. Currently, there are about 4.5 million individuals with a cannabis SUD, 1 million with methamphetamine, another 1 million with cocaine use disorder (SAMHSA, 2019). Therefore, an approved medication for any of these disorders will be an important public health contribution and a unique market opportunity for a pharmaceutical company committed to this field.
For stimulant use disorders (cocaine and methamphetamine) after many years of research and large amounts of time and funds invested, there is no successful pharmacotherapy approved by regulatory agencies. Multiple approaches, targets, medications, and biologics have been tried. The most common pharmacological target has been the dopaminergic system, with disappointing results. Other systems such as the noradrenergic, serotonergic, glutamatergic, GABAergic, etc., have been targeted and the results have also been disappointing. More recently, biologics such as vaccines, monoclonal antibodies, and engineered enzymes have been tested, with similar results. Unfortunately, currently there are no medications in Phase III clinical trials for these disorders and we may be years away from having any pharmacological treatment approved. This is an area where more collaboration among industry, academics, and government agencies is urgently needed (Montoya, 2012; Montoya & McCann, 2010; Ronsley et al., 2020).
«Anti-drug vaccines produce an immunological response characterized by the production of antibodies against the specific drug of abuse»
For cannabis use disorders, there is controversy about the need of treatments given the generalized public perception of low risk of cannabis use. However, as it has been reported in the NSDUH of 2019, almost 5 million individuals in the U.S. met criteria and 827,000 people were treated for this disorder. Moreover, enough research has been carried out to confirm that the chronic use of cannabis can produce serious physical and psychological consequences, including cannabis withdrawal syndrome. Therefore, there is a clear need to develop medications to treat the disorder. Several medications have been investigated, some of them targeting the whole disorder and others some specific aspects, for example, sleep disturbances, withdrawal syndrome, reduction reinforcing effects, and relapse prevention. Most tested medications can be two groups: 1) those that have their effect on the cannabinoid system (e.g., cannabinoid agonists, partial agonists, agonists, etc.) and 2) those that have their effect on other neurotransmitter systems (e.g., serotoninergic, GABAergic, noradrenergic, etc.). Unfortunately, the efforts to find a safe and effective medication for cannabis use disorder have not been successful. However, NIDA in partnership with academic and industry investigators continue the search for safe and effective pharmacological interventions for this disorder (Montoya & Weiss, 2018; SAMHSA, 2019; Vandrey & Haney, 2009).
Given the current opioid use epidemic in the United States, efforts have significantly increased to support the development of safer and more effective medication for opioid use disorder. This effort has been channeled through the Helping to End Addiction Long Term (HEAL) initiative of the National Institutes of Health (NIH). Currently more than 40 new compounds and medications are being evaluated under this program. They include small molecules and biologics to prevent or treat opioid use disorder and overdose. The goal is to enhance the armamentarium of options for clinicians to treat these conditions (Collins et al., 2018).
Conclusions
The development of medications to prevent or treat SUD and drug overdose is a challenging and expensive process that often does not result in regulatory approval. A new compound may fail because of medical safety concerns or lack of efficacy. In addition to the usual medical safety risks, compounds for SUD may fail because of potential abuse liability and iatrogenically inducing a new drug addiction, as well as adverse interactions with drugs of abuse (e.g., increase of respiratory depression of opioids or cardiovascular toxicity of stimulants).
«The development of medications to treat substance use disorder is a challenging and expensive process that often does not result in regulatory approval»
Multiple efforts by academic, industry and government investigators have resulted in the regulatory approval of pharmacotherapies for opioid use disorder and overdose. However, there is room to improve their safety and efficacy. Initiatives by the U.S. government, like the NIH HEAL Initiative and NIDA’s Medications Development Program, in partnership with academic and industry investigators, are boosting the research and development of medications for SUD. The expectation is that in few years more medications will be approved, which will enhance the therapeutic arsenal to significantly reduce the public health burden of SUDs and improve the quality of life of those suffering it.
On a final note, the COVID-19 pandemic has had devastating consequences on SUD and the progress of its medication’s development research (Volkow, 2020). The temporary shut-down of animal research laboratories has significantly delayed the pre-clinical testing of new compounds and for clinical research the situation has been worse. Many institutional review boards ordered stopping recruitment of new study participants in clinical trials and those that were recruited before the pandemic were only allowed to remain in the studies for clinical care with minimal collection of data. In addition, some of the study methods, laboratory tests, and assays had to be adapted to accommodate the COVID-19 priorities. Moreover, the FDA had to prioritize all COVID-19 related regulatory submissions, and approvals for non-COVID-19 related medications are significantly delayed. Therefore, the progress in medications development for SUD that had been made before the pandemic slowed down and it is likely that the availability of new medications for patients will delayed. After the pandemic is over or we learn to live with the virus, the hope is that medications development will be accelerated and research can recuperate some of the time that was lost, and safe and effective medications to treat SUD will be available in the near future.
References
Banks, M. L., Townsend, E. A., & Negus, S. S. (2019). Testing the 10 most wanted: A preclinical algorithm to screen candidate opioid use disorder medications. Neuropsychopharmacology, 44(6), 1011–1012. https://doi.org/10.1038/s41386-019-0336-5
Coffa, D., & Snyder, H. (2019). Opioid use disorder: Medical treatment options. American Family Physician, 100(7), 416–425.
Collins, F. S., Koroshetz, W. J., & Volkow, N. D. (2018). Helping to end addiction over the long-term: The research plan for the NIH HEAL Initiative. JAMA, 320(2), 129–130. https://doi.org/10.1001/jama.2018.8826
Epstein, D. H., Preston, K. L., & Jasinski, D. R. (2006). Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: Lessons from tramadol. Biological Psychology, 73(1), 90–99. https://doi.org/10.1016/j.biopsycho.2006.01.010
Johnson, D. N., & Vocci, F. J. (1993). Medications development at the National Institute on Drug Abuse: Focus on cocaine. NIDA research monograph, 135, 57–70.
Kiluk, B. D., Carroll, K. M., Duhig, A., Falk, D. E., Kampman, K., Lai, S., Litten, R. Z., McCann, D. J., Montoya, I. D., Preston, K. L., Skolnick, P., Weisner, C., Woody, G., Chandler, R., Detke, M. J., Dunn, K., Dworkin, R. H., Fertig, J., Gewandter, J., … Strain, E. C. (2016). Measures of outcome for stimulant trials: ACTTION recommendations and research agenda. Drug and Alcohol Dependence, 158, 1–7. https://doi.org/10.1016/j.drugalcdep.2015.11.004
Kleykamp, B. A., De Santis, M., Dworkin, R. H., Huhn, A. S., Kampman, K. M., Montoya, I. D., Preston, K. L., Ramey, T., Smith, S. M., Turk, D. C., Walsh, R., Weiss, R. D., & Strain, E. C. (2019). Craving and opioid use disorder: A scoping review. Drug and Alcohol Dependence, 205, 107639. https://doi.org/10.1016/j.drugalcdep.2019.107639
Kreek, M. J., Reed, B., & Butelman, E. R. (2019). Current status of opioid addiction treatment and related preclinical research. Science Advances, 5(10), eaax9140. https://doi.org/10.1126/sciadv.aax9140
Loflin, M. J. E., Kiluk, B. D., Huestis, M. A., Aklin, W. M., Budney, A. J., Carroll, K. M., D’Souza, D. C., Dworkin, R. H., Gray, K. M., Hasin, D. S., Lee, D. C., Le Foll, B., Levin, F. R., Lile, J. A., Mason, B. J., McRae-Clark, A. L., Montoya, I., Peters, E. N., Ramey, T., … Strain, E. C. (2020). The state of clinical outcome assessments for cannabis use disorder clinical trials: A review and research agenda. Drug and Alcohol Dependence, 212, 107993. https://doi.org/10.1016/j.drugalcdep.2020.107993
Montoya, I. D. (2012). Advances in the development of biologics to treat drug addictions and overdose. Adicciones, 24(2), 95–103. https://doi.org/10.20882/adicciones.101
Montoya, I. D. (Ed.) (2016). Biologics to treat substance use disorders. Springer International Publishing. https://doi.org/10.1007/978-3-319-23150-1
Montoya, I. D., Hess, J. M., Preston, K. L., & Gorelick, D. A. (1995). A model for pharmacological research-treatment of cocaine dependence. Journal of Substance Abuse and Treatment, 12(6), 415–421. https://doi.org/10.1016/0740-5472(95)02017-9
Montoya, I. D., & McCann, D. J. (2010). Drugs of abuse: management of intoxication and antidotes. In A. Luch (Ed.), Molecular, Clinical and Environmental Toxicology. Experientia Supplementum, vol 100, (pp. 519–541). Birkhäuser Basel. https://doi.org/10.1007/978-3-7643-8338-1_15
Montoya, I. D., & Weiss, S. (Eds.) (2018). Cannabis use disorders. Springer International Publishing. https://doi.org/10.1007/978-3-319-90365-1
Rasmussen, K., White D. A., & Acri, J. B. (2019). NIDA’s medication development priorities in response to the Opioid Crisis: Ten most wanted. Neuropsychopharmacology, 44(4), 657–659. doi: https://doi.org/10.1038/s41386-018-0292-5
Ronsley, C., Nolan, S., Knight, R., Hayashi, K., Klimas, J., Walley, A., Wood, E., & Fairbairn, N. (2020). Treatment of stimulant use disorder: A systematic review of reviews. PLOS One, 15(6), e0234809. https://doi.org/10.1371/journal.pone.0234809
SAMHSA. (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Center for Behavioral Health Statistics and Quality. Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data
Skolnick, P., & Volkow, N. D. (2012). Addiction therapeutics: obstacles and opportunities. Biological Psychiatry, 72(11), 890–891. https://doi.org/10.1016/j.biopsych.2012.08.004
Vandrey, R., & Haney, M. (2009). Pharmacotherapy for cannabis dependence: How close are we? CNS Drugs, 23(7), 543–553. https://doi.org/10.2165/00023210-200923070-00001
Vocci, F., & Ling, W. (2005). Medications development: Successes and challenges. Pharmacology and Therapeutics, 108(1), 94–108. https://doi.org/10.1016/j.pharmthera.2005.06.010
Volkow, N. D. (2020). Collision of the COVID-19 and addiction epidemics. Annals of Internal Medicine, 173(1), 61–62. https://doi.org/10.7326/m20-1212
Yu, E., Miotto, K., Akerele, E., Montgomery, A., Elkashef, A., Walsh, R., Montoya, I., Fischman, M. W., Collins, J., McSherry, F., Boardman, K., Davies, D. K., O’Brien, C. P., Ling, W., Kleber, H., & Herman, B. H. (2008). A Phase 3 placebo-controlled, double-blind, multi-site trial of the alpha-2-adrenergic agonist, lofexidine, for opioid withdrawal. Drug and Alcohol Dependence, 97(1-2), 158–168. https://doi.org/10.1016/j.drugalcdep.2008.04.002