From crayfish to humans
An evolutionary perspective of addiction
Addiction is a complex disease whose manifestation is unique to each individual patient. Despite this, our knowledge suggests that many of the consequences of using drugs of abuse are due to alterations in the brain, which would be similar from one individual to another. Specifically, drugs of abuse act on the brain’s reward system to trigger behavioural effects. In this paper, we will unravel the functions and phylogenetic roots of this system and then explain how drugs of abuse can affect the functioning of the brain. Addiction research and treatment requires a biopsychosocial approach and hence, being aware of the phylogenetic side of this problem can help to build a holistic view of the disease.
Keywords: addiction, evolution, brain reward system, drugs of abuse, phylogenetic perspective.
drugs of abuse: an interspecific problem
Addiction can be defined as a chronic, recurrent brain disease, characterised by compulsive drug seeking and use regardless of the negative consequences to the individual (National Institute on Drug Abuse [NIDA], 2008). However, despite the wide consensus to the contrary in scientific and academic circles, the popular view remains that addiction is a problem of willpower and that addicts simply «don’t try hard enough» to stop using. While such views are rare in the professional field, one can still find academic texts claiming that addiction is a mental disorder with purely psychological determinants. Some even go so far as to speak of addictive personality disorders. According to this view, addiction would be a uniquely human phenomenon originating in an error at the level of our highest cognitive capacities.
However, it seems that we are not the only ones who have «drug problems». For instance, invertebrate animals have much simpler nervous systems and they, nonetheless, exhibit behaviours remarkably similar to those found in human responses to drugs (Van Staaden et al., 2018). Studies involving crustaceans such as lobsters and crayfish (Figure 1), for example, have found that these animals show behavioural disinhibition and motor disturbances analogous to those seen in humans during the intoxication phase of the action of drugs such as cocaine, amphetamines, or alcohol (Nathaniel et al., 2010; Van Staaden et al., 2018), as well as tolerance and sensitisation to these effects after repeated administrations.

Figure 1. Some crustaceans exhibit a response to alcohol that resembles alcohol intoxication or «binge drinking» in humans. Norway lobsters and crayfish swimming in a tank containing alcohol show motor disturbances such as an inability to move in a straight line and difficulty in maintaining posture. With repeated exposure to alcohol, these species develop tolerance, showing increasingly rapid recovery of motor control after consumption. / Photo: Hans Hillewaert
Just like in humans, these invertebrates appear to experience drug use as pleasurable and self-reinforcing. Specifically, lobsters and crayfish have been observed to develop a preference for the contexts in which they received a drug (Nathaniel, et al., 2010; Van Staaden et al., 2018). In addition, they were also motivated to learn and perform simple operational behaviours to obtain a new dose of these substances (Datta et al., 2018). Further analogies to the addictive process in humans suggest that these learning and associative memories, linked to the reinforcing power of drugs, are likely to be extinguished after pairings with the conditioned stimulus without drug administration, but reappear when the drug is readministered (Nathaniel et al., 2010). All of this suggests that these animals exhibit a vulnerability to relapse similar to that of mammals such as humans.
But how is it possible for creatures that are so different from humans to display behaviours analogous to our own actions in response to drugs? Perhaps adopting an evolutionary view of addiction could help us answer this question.
An evolutionary perspective of addiction
Drugs of abuse can modulate behaviour through their actions on the nervous system, specifically on a brain circuit called the reward system (NIDA, 2008). This system has very ancient phylogenetic roots and has been preserved over the centuries in a variety of animal species, both invertebrates and vertebrates, including humans (Durrant et al., 2009).
«Drugs of abuse can modulate behaviour through their actions on the nervous system»
From a Darwinian perspective, the reason why this system has been retained in vastly different species living in such disparate environments is very easy to understand. This system allowed both lobsters and humans to adapt better to their environment, because it was responsible for promoting and maintaining behaviours that were basic for their ancestor’s survival, such as obtaining food, sex, or encouraging affiliative and social behaviour (Nesse & Berridge, 1997). Therefore, the brain reward system we share with other animals is phylogenetically very old. It is the substrate upon which drugs of abuse exert their effects (Figure 2). After all, several of these substances with psychoactive properties were initially obtained from the natural world. While synthetic drugs are now available, they are merely laboratory reproductions and modifications of the molecular structures present in these natural substances.
So why can substances that are external to our bodies modulate this system and induce such dramatic addictive effects? When it comes to answering this question, there are two distinct schools of thought.
Figure 2. An evolutionary perspective of addiction. The reward system (shown in blue) is a brain circuit with very ancient phylogenetic roots which is responsible for regulating the processes necessary to ensure that the behaviours that are basic for survival are maintained. Drugs of abuse can interfere with this system, thus compromising the survival of the individual. / Source: Authors
The first suggests that consuming these natural psychoactive substances would have clear adaptive benefits for humans, such as the ability of some psychostimulants to reduce fatigue and appetite, or alcohol to reduce anxiety (Hagen et al., 2013). These benefits would explain why humans have evolved and developed a system adapted to seek out and consume these substances. However, this view also has its detractors, who consider that the evidence for the existence of a regulatory system specifically for the consumption of these substances is insufficient because we lack a taste and olfactory preference system to ensure they are sought out and consumed (Durrant et al., 2009).

Figure 3. Some plants, fungi, and other living organisms produce psychoactive substances that they use to defend against predators. Nicotine, for example, is an alkaloid produced by the tobacco plant to repel insects. However, this substance also has psychoactive effects that have been studied and exploited by humans. / Photo: Markus Distelrath
The second school of thought suggests that the effects of these psychoactive substances (and of all drugs of abuse) on the brain can ultimately be explained as a side effect of evolution (Van Staaden et al., 2018). According to this view, these psychoactive substances would be produced by plant organisms, fungi, and other living things to modulate the behaviour of potential predators. Some plants, for example, produce chemicals called secondary metabolites, which fulfil multiple non-vital functions such as attracting pollinating insects or defending against herbivores (Wink, 2018). These secondary plant metabolites would therefore have the potential to modify the behaviour of some of the animals that consume them, mainly insects (Figure 3). This is possible because at the molecular level, the structure of these substances is remarkably similar to neurotransmitters such as serotonin, dopamine, and endorphins, among others (Wink, 2018), and so they can interfere with the functioning of the nervous system of the animal that consumed them.
More specifically, psychoactive substances that are used as drugs of abuse would be able to «short-circuit» the brain’s reward system which, as we have already mentioned, regulates the performance of basic survival behaviours. They induce a false signal announcing the arrival of a great benefit for our survival. This false signal is so aberrant that it ends up altering the normal functioning of our reward system, which then prioritises obtaining the drug over any other vital resource, despite the negative consequences derived from its consumption. In the following section, we will delve deeper into the functioning of the brain’s reward system and how drugs «hijack» it.
The brain’s reward system: the target of drugs of abuse
The brain’s reward system regulates motivational and learning processes aimed at the search for and consumption of resources that allow the survival of individuals and their species (Nesse & Berridge, 1997). To this end, the reward system performs two basic functions: establishing a hedonic value (liking) for the resources we interact with and promoting the need, searching, and consumption behaviours associated with stimuli that have been beneficial in the past (wanting) (Robinson & Berridge, 1993; Robinson et al., 2015). Anatomically, these functions are supported by a circuit that starts in the ventral tegmental area and projects to the nucleus accumbens and prefrontal cortex (Figure 4). This circuit also includes connections with other structures such as the hippocampus, amygdala, olfactory tubercle, and lateral septal nucleus (Goldstein & Volkow, 2011).
«Drugs activate the reward system, promoting a misleading signal that announces the arrival of a huge benefit for survival»
As part of our interaction with the environment, when we behave in a way that favours our survival, such as drinking water, eating, or resting, we tend to feel a positive or «pleasurable» sensation that corresponds to the first function of the brain’s reward system (liking). This response reinforces the behaviour, that is, it increases the probability that behaviours that have previously proved to be pleasurable are repeated and promotes avoidance and escape from experiences resulting in aversion. At the physiological level, encoding this response is achieved by an increase (spike) in the release of dopamine and other substances such as the GABA neurotransmitter and endorphins (Nutt et al., 2015).
However, to ensure our survival over time, besides discriminating between beneficial and harmful stimuli, we must learn to discriminate when resources are available and generate the desire and motivation to obtain them at that time. This task corresponds to the second function of the reward system (wanting), which tries to ensure that beneficial resources are obtained in the future. To this end, apart from encoding the hedonic value of a particular stimulus, activation of the reward system triggers learning processes. In turn, these processes provide «salience» to beneficial stimuli, so that when the resources are present in a given context, they are perceived as relevant and attract our attention. In addition, the reward system also gives them an incentive value, i.e., the ability to motivate approach and consumption behaviours (Robinson & Berridge, 1993). This learning is not limited to a specific resource or stimulus, but rather extends to the rest of the stimuli or contextual cues with which it was presented. Thus, we learn to seek and want a resource – food, for instance – not only when it is present, but also when we are in a context that was associated with the availability of that resource in the past (Durrant et al., 2009). This means that when we walk through the door of our favourite restaurant, we feel a certain «urge» or desire to enter, especially if we happen to be hungry at the time.
However, even if we do feel that desire, we do not always respond with the consummatory behaviour of walking in and asking for a table at the restaurant. This is because the ultimate control of conscious behaviour is regulated by other brain systems, such as the prefrontal cortex, which monitors and regulates the behaviour promoted by the reward system (Robinson et al., 2015). Thus, even though the pleasure and desire responses promoted by the reward system are automatic, consummatory behaviours are supervised by higher cortical structures that aim to ensure we select the behaviour with the best cost-benefit balance in our interaction with the environment. That is, we are not always driven by the impulse to get immediate reinforcement; we may decide not to go to our favourite restaurant because we have a business lunch we cannot miss, or simply because we want to save money.
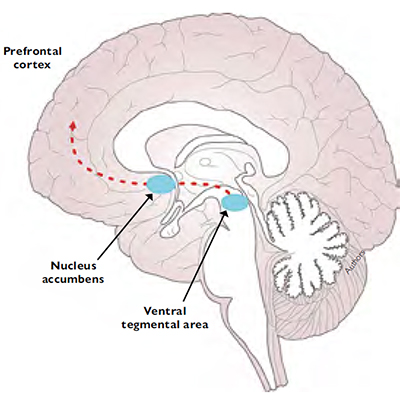
Figure 4. The brain reward system is a circuit that corresponds to the cortical-mesolimbic dopaminergic pathway. This pathway includes projections that start in the ventral tegmental area and end in the nucleus accumbens, connecting with other structures such as the prefrontal cortex, which regulates the behaviour promoted by the reward system. In addition to «hijacking» or «hacking» this system, repeated drug use leads to a disconnection with the prefrontal structures that would normally curb harmful behaviour.
However, as we have discussed, drugs of abuse can hijack or «hack» the functioning of these systems. Regardless of their mechanism of action, these drugs activate our reward system and thereby promote a misleading signal that announces the arrival of a huge benefit for our survival (Nesse & Berridge, 1997). They do this by dramatically increasing the release of certain neurotransmitters – mainly dopamine – in the brain’s reward circuitry. Thus, after using a drug of abuse, there is a huge dopamine spike three to tenfold greater than the response to natural reinforcers such as sex or food (Wightman & Robinson, 2002; Wise, 2002). Following continued drug use, these spikes in dopamine and other neurotransmitters, which are way beyond natural levels, will lead to physiological and functional alterations in the reward system, resulting in behavioural changes that can lead to addiction. In general, addicted individuals appear to be less responsive to natural reinforcers. This is because they lose value compared to the reinforcing signal provided by drugs of abuse, which become the only source of «pleasure» for the individual. In addition to these alterations in the person’s liking mechanisms, we can also observe an exaggerated motivation to seek out and consume the drug. This consumption develops in an uncontrolled and impulsive manner, guided by drug-related cues and stimuli that hijack the addict’s attention and promote an uncontrollable desire to consume the substance. Finally, consummatory behaviours are carried out regardless of the negative impact they may have on the person’s overall functioning; after repeated contact with the drug, there is a disconnection with the prefrontal structures that should curb such harmful behaviour (Goldstein & Volkow, 2011).
It should be noted that not everyone who is repeatedly exposed to a drug like alcohol will eventually progress to addiction. The onset of addiction is modulated by a number of environmental variables and individual differences, both biological and psychological, that condition vulnerability or a predisposition to develop the disorder (Wakefield, 2020). Hence, much of the current research in the field of addiction is aimed at studying which environmental and biological factors are risk factors for the development of addictive disorders as well as which interventions can mitigate or neutralise their impact.
Conclusion
Addiction is a brain disease whose study and treatment requires a biopsychosocial approach in which genetic, environmental (e.g., stress), and social determinants must be considered. An anthropocentric attitude towards the study and treatment of addiction would lead us to ignore all the advances made so far in preclinical research (with animal models) and in clinical research, which have made it possible, for example, to develop new pharmacological and psychological treatments. Although the manifestations and consequences of the disorder are unique in each person, the scientific knowledge accumulated to date suggests that many of the behavioural effects of drugs are due to alterations that occur in the brain (neuroadaptations), and that these are similar across individuals. These alterations are found in a circuit that is fundamental for survival, both of the individual and of the species, and therefore all potential therapeutic strategies must take this fact into account. In other words, they should adopt a holistic perspective of this disease.
References
Datta, U., Van Staaden, M., & Huber, R. (2018). Crayfish self-administer amphetamine in a spatially contingent task. Frontiers in Physiology, 9, 433. https://doi.org/10.3389/fphys.2018.00433
Durrant, R., Adamson, S., Todd, F., & Sellman, D. (2009). Drug use and addiction: Evolutionary perspective. Australian & New Zealand Journal of Psychiatry, 43(11), 1049–1056. https://doi.org/10.3109/00048670903270449
Goldstein, R. Z., & Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews Neuroscience, 12(11), 652–669. https://doi.org/10.1038/nrn3119
Hagen, E. H., Roulette, C. J., & Sullivan, R. J. (2013). Explaining human recreational use of ‘pesticides’: The neurotoxin regulation model of substance use vs. the hijack model and implications for age and sex differences in drug consumption. Frontiers in Psychiatry, 4, 142. https://doi.org/10.3389/fpsyt.2013.00142
Nathaniel, T. I., Panksepp, J., & Huber, R. (2010). Effects of a single and repeated morphine treatment on conditioned and unconditioned behavioral sensitization in crayfish. Behavioural Brain Research, 207(2), 310–320. https://doi.org/10.1016/j.bbr.2009.10.010
National Institute on Drug Abuse. (2008). Drugs, brain, and behavior: The science of addiction. NIDA, National Institute of Health.
Nesse, R. M., & Berridge, K. C. (1997). Psychoactive drug use in evolutionary perspective. Science, 278(5335), 63–66. https://doi.org/10.1126/science.278.5335.63
Nutt, D. J., Lingford-Hughes, A., Erritzoe, D., & Stokes, P. R. (2015). The dopamine theory of addiction: 40 years of highs and lows. Nature Reviews Neuroscience, 16(5), 305–312. https://doi.org/10.1038/nrn3939
Robinson, M. J. F., Fischer, A. M., Ahuja, A., Lesser, E. N., & Maniates, H. (2015). Roles of “wanting” and “liking” in motivating behavior: Gambling, food, and drug addictions. En E. Simpson, & P. Balsam (Eds.), Behavioral neuroscience of motivation. Current topics in behavioral neurosciences, vol. 27 (p. 105–136). Springer. https://doi.org/10.1007/7854_2015_387
Robinson, T. E., & Berridge, K. C. (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews, 18(3), 247–291. https://doi.org/10.1016/0165-0173(93)90013-P
Van Staaden, M. J., Hall, F. S., & Huber, R. (2018). The deep evolutionary roots of ‘addiction’. Journal of Mental Health and Clinical Psychology, 2(3), 8–13. https://doi.org/10.29245/2578-2959/2018/3.1135
Wakefield, J. C. (2020). Addiction from the harmful dysfunction perspective: How there can be a mental disorder in a normal brain. Behavioural Brain Research, 389, 112665. https://doi.org/10.1016/j.bbr.2020.112665
Wightman, R. M., & Robinson, D. L. (2002). Transient changes in mesolimbic dopamine and their association with ‘reward’. Journal of Neurochemistry, 82(4), 721–735. doi: https://doi.org/10.1046/j.1471-4159.
2002.01005.x