A passion for chemistry
Historical and current references in recreational chemistry and implications for teaching and outreach
After introducing some general ideas about recreational chemistry, here I provide different historical references from the 18th century to the present day. These include philosophical evenings, the creation of institutions for popularising science, and the publication of emblematic books and journals on the subject. I discuss the activities of some prominent personalities in recreational chemistry and analyse the specificities of different aspects of this domain such as varying approaches (games and gamification, science fairs, media, the internet, and software, etc.). These are compared to other fields of science popularisation (prevention and safety, specific recommendations for its use, etc.) and implications for science teaching and popularisation in relation to this field of knowledge. Finally, with the conclusions, I have also provided bibliographical references to facilitate further study of the subject.
Keywords: science education, science popularisation, recreational chemistry, chemistry and everyday life.
Introduction to recreational chemistry
Recreational science is generally considered to be the part of the dissemination and popularisation of science which, in addition to the usual educational and training purposes, promotes fun in some way, either for the person doing the activity individually or in a small group, or (most importantly) for the participating public.
In this paper I provide general reflections on recreational science, specifically regarding the field of chemistry, which in many cases (traditionally) overlaps with physics and other branches of science. After introducing some historical references, I describe some ways of approaching recreational chemistry, some inherent characteristics of the activities involved – with specific examples – and I then analyse their implications for the dissemination and popularisation of this science.
Historical references of recreational chemistry in Europe
As García Molina (2011) pointed out, in the 18th and 19th centuries, scientific exhibitions became very popular in street demonstrations and popular fairs. There were also scientific evenings (initially called philosophical evenings), similar to literary and musical evenings, where the bourgeoisie gathered to keep abreast of the latest developments in all fields. Literary and philosophical salons originated in France in the 17th century, but soon spread throughout Europe (Kale, 2004). They were often sponsored by women (salonnières), such as Marie-Anne Pierrette Paulze (1758–1836), the widow of Antoine-Laurent de Lavoisier (1743–1794), one of the founders of modern chemistry.
At the end of the 18th century, during the Age of Enlightenment, practical chemistry was very popular in enlightened circles. In Paris alone, nearly 300 laboratories were identified in private homes, with another 200 in pharmacies, academic institutions, and in industry (Garcia Belmar, 2020). During the 19th century, the scientific and mathematical recreations of the 18th century that had contributed to the dissemination of science were often referred to as science amusante and aimed to «educate while entertaining» (Hache-Bissette, 2017).
The Royal Institution of Great Britain was founded in 1799 to disseminate scientific knowledge and intriguing experiments (Berman, 1972). It still exists today in its original building in London’s Albemarle Street and has maintained its commitment to science through its ongoing activities. Personalities such as Humphry Davy (1778–1829), Michael Faraday (1791–1867), and William Lawrence Bragg (1890–1971) all worked there. As well as popularising science, important scientific work was also carried out there, including the discovery or isolation of 10 different chemical elements. Indeed, among the many other events held there are the Christmas Lectures, aimed at children, which have been given since 1825. In fact, in one of his landmark lectures there, «The chemical history of a candle», Faraday explained some principles of physics and chemistry while making observations about something as mundane as burning of a candle. Today, the building (Figure 1) houses a museum about Faraday, recreating some of his research.

Figure 1. In the first image, the façade of the Royal Institution building by Thomas Hosmer Shepherd (ca. 1838). The institution was founded in 1799 to disseminate scientific knowledge and curious experiments, and its activity continues to this day. In the second image, a lecture by the English physicist and chemist Michael Faraday, in the presence of Prince Albert (Queen Victoria’s consort) and his children, at the Royal Institution. Wood engraving based on the work of Alexander Blaikley (ca. 1856). / Public domain / Wellcome Collection / Public domain / The Royal Institution
Classics in recreational chemistry
A regular visitor to the Royal Institution was Jane Marcet – née Haldimand – (1769–1858) (Figure 2), who was popular in her day as an author of introductory books on science and other subjects such as economics and plant physiology. Some of her texts, aimed at young women (who were generally less educated than men at the time), take the form of conversations between two schoolgirls, Caroline (who is curious and disbelieving) and Emily (the more rational and industrious of the two) and their teacher (Mrs. Bryan). Her book, Conversations on chemistry, intended more especially for the female sex, which she published anonymously in 1805, is notable for explaining chemistry in a familiar way, illustrating it with experiments (Crellin, 1979). Attentive to new discoveries, she revised the book in subsequent editions, although she did not acknowledge her authorship until the 12th edition (1832). As Eva Armstrong (1938) pointed out, the work of Jane Marcet contributed greatly to the popularisation of chemical knowledge and understanding in several countries, especially among the female readers for whom the book was intended,1 and even inspired (by his own admission) the young Michael Faraday, who had no formal education.
However, Jane Marcet’s work was not unique. The first known treatise on chemistry written by a woman, Marie Meurdrac (c. 1610–1680) and intended for women, was published in 1666. In La chymie charitable et facile en faveur des dames (‘Charitable and easy chemistry in favour of ladies’), she combined her knowledge of the chemistry of the time with her experience in making cosmetics, medicines, and ointments, some of which she gave away free to the poor – hence the adjective charitable.
Another classic book, Les récréation scientifiques (‘Science recreations’) (Figure 3), was written in the second half of the 19th century and has many editions and translations. The book presented physical and chemical experiments using everyday materials and stated that science, as well as being used to satisfy the necessities of life, «can also be the subject or theme of various very pleasant entertainments or pastimes» (Tissandier, 1880/1884). The author, the Frenchman Gaston Tissandier (1843–1899), was interested in aerostation and made dozens of ascents. A hot-air balloon is even engraved on his gravestone! Tissandier was passionate about popularising science and wrote other books such as La Tour Eiffel de trois cents mètres, which described the construction of the monument. He also founded the popular scientific journal La Nature (Figure 3), which was published between 1873 and 1896. Other popular books of the 19th century were those of Parkes (1819) and Richard (1856). In the former, the author states: «Nothing tends to imprint chemical facts upon the mind so much as the exhibition of interesting Experiments».
Some emblematic books on recreational chemistry in more recent times include The golden book of chemistry experiments (Figure 4), published in 1960, which was challenged only a decade later because many of the reactions described produced toxic or corrosive products. A more recent recreational chemistry book is that of the Lebanese American Professor Bassam Z. Shakhashiri (b. 1939) (Figure 5), which he has disseminated in hundreds of conferences, schools, and shopping centres, as well as on television, etc., including a four-decade-long programme of «Christmas Science» demonstrations reminiscent of those of Faraday. His book, Chemical demonstrations: A handbook for teachers of chemistry, is structured into five volumes and has been continuously edited since 1983. Similar to the work of Shakhashiri, it is also important to mention Martyn Poliakoff (b. 1947) (Figure 5), an English contemporary populariser of science who has been very popular over the last two decades, partly because of his hundreds of entertaining videos on chemical elements and the periodic table (Haran & Poliakoff, 2011).
Historical references of recreational chemistry in Spain
Spain also had a taste for recreational science. For example, the Frenchman Joseph Louis Proust (1754–1826), who worked for several years as a professor of chemistry in Spain, carried out several demonstrations of hot-air balloons (an activity developed by chemists at the time as a practical application of the properties of gases). Another example is the creation in 1816, of the Gabinete de Física y Química de Palacio in Madrid, which was dedicated to the recreation of the royal family and to public instruction (Puerto Sarmiento, 1994). Moreover, in an advertisement published in El Diario de Madrid in 1837, a «servant» offered his services to, among other things, «teach children a lot of recreational and curious chemistry» (Figure 6).
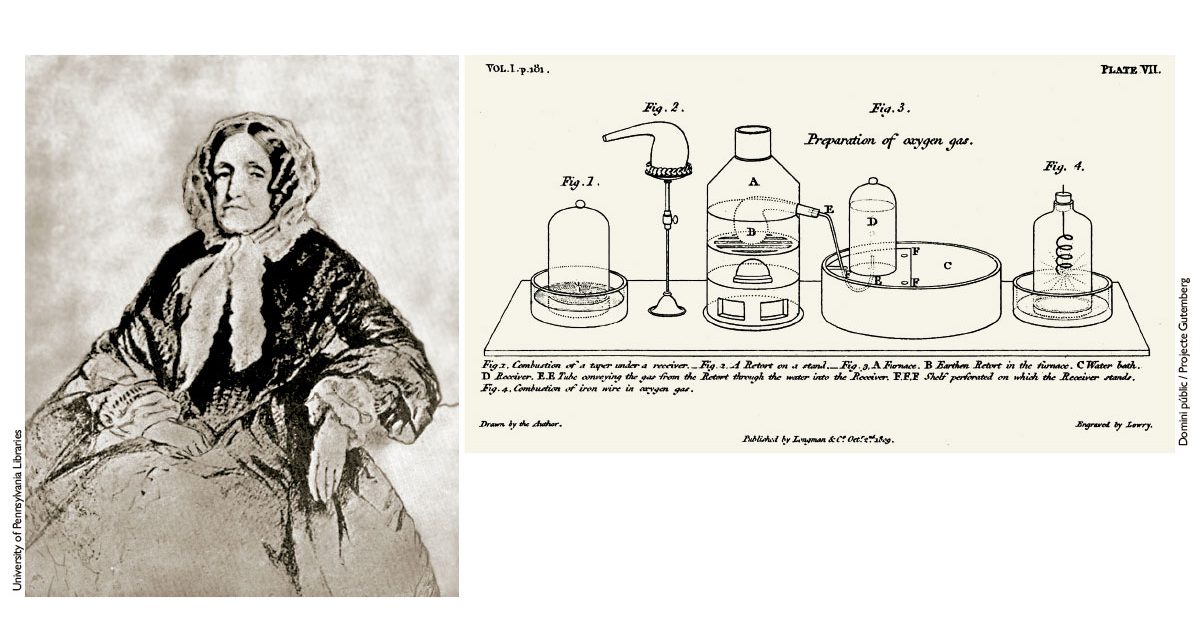
Figure 2. On the left, portrait of Jane Marcet, a pioneer in the popularisation of science for women. She published her book, Conversations on chemistry, anonymously in 1806, for which she also produced illustrations such as the one shown above these lines. / University of Pennsylvania Libraries/ Public domain / Project Gutemberg
As an example of the usefulness of «amusing chemistry» for the «brilliant education of the young», Vélez de Paredes explained, in another emblematic text from the mid-19th century, that «even the well-to-do and affluent will find here a useful and amusing past-time that will brighten up their long hours of leisure and put them in a position where they can stand out in scientific, artistic, and industrial circles» (Vélez de Paredes, 1860). In the same book, he points out that men «commonly feel a certain detachment, and sometimes antipathy, to laborious, serious, and complicated studies» and suggests that his book will overcome laziness in study by making chemistry «pleasant and delightful». In the first hundred or so pages, he offers «general notions for learning chemistry without a teacher» (including a table of the 64 simple substances known at the time and details on the organisation of an inexpensive laboratory). In the second chapter, which is nearly 200 pages long, he presents «useful and amusing experiments», including multiple chemical reactions (such as combustions and precipitations) and experiments to obtain «amusing» coloured inks (such as «invisible» ink), gunpowder, and to fake precious stones, etc. Each experiment could be carried out using «everyday» materials of the time (e.g., camphor and illuminating gas, etc.) and, most importantly, he discusses the causes of each phenomenon, rather than describing mere curiosities.
Decades later, in 1918, José Estalella published Ciencia recreativa: enigmas y problemas, observaciones y experimentos, trabajos de habilidades y paciencias (‘Recreational science: puzzles and problems, observations and experiments, skill and patience work’). In addition to the detailed experiments in chemistry and other sciences that it provides, his book was also notable because it included up to date (for the time) international references.
Approaches to recreational chemistry
Many teachers and researchers assume that society in general, as well as students at different stages of education, have a certain negative image of chemistry, perhaps seeing it as useless, dangerous, polluting, and only for laboratories and industry. As I write this paper, I am not so «pessimistic»: there are survey results that point to a high general public appreciation of science in general and, in my personal perception, of chemistry in particular (including biochemistry). However, it is true that, compared to other sciences, the collectives involved in chemistry tend to try to compensate for the sometimes distorted image of the field and thus, a wide variety of initiatives are in place to make «chemistry fun».
Apart from books (Pinto et al., 2006), articles in journals, conferences, and exhibitions by science popularisers, there are many other formats that promote recreational chemistry, which I will briefly mention and explain below.
First, science fairs are increasingly widespread in schools and at the local, regional, national, and international levels, in many formats. Among other local examples in Spain, Madrid es Ciencia, Ciència al Carrer (Lleida), Diverciencia (Algeciras, Cádiz), and the Encuentro de Ciencias Bezmiliana (Rincón de la Victoria, Málaga) are all well established. At the national level, Ciencia en Acción, which has been held every year since the year 2000 and has around 20 different modalities, stands out, as does its European counterpart, Science on Stage.

Figure 3. A) Front cover of the journal La Nature, created by G. Tissandier, and some plates from his emblematic book Les récréations scientifiques (‘Science recreations’); B) boiling water in a paper cup; C) the «pharaoh’s snake», a type of firework; D) «fun» experiment with hydrochloric acid and ammonia to create smoke inside a stoppered cup; and E) melting a piece of tin into a playing card. / Internet Archive
There are also many student competitions in different formats. One of these is the MasterChem competition, which has been organised for several years by the University of Murcia, with a format similar to the popular TV cooking show MasterChef. The competition adds a festive atmosphere to the rigour of the competition, unlike other more conventional competitions and championships, such as the Chemistry Olympiad.
Similarly, there are many leisure activities such as the Pint of Science festival, first organised in 2012 by researchers at Imperial College London, which has now spread to many countries, including Spain. The aim of this festival is to offer interesting and entertaining talks on the latest scientific research in a format accessible to the general public; the most original aspect is that the talks take place in pubs. Another activity of this type, also from the UK, is the FameLab, a Talking Science monologue competition. Known in Spain as Solo de Ciencia, it is an emblematic activity that has also inspired other similar events (such as three-minute dissertation presentations). In addition, there are an increasing number of relaxed outreach events on symbolic dates (e.g., the International Day of Women and Girls in Science and Science Week, etc.), where some kind of chemistry «show» is always a given.
Supported by the European Commission as part of the Marie Skłodowska-Curie Actions of the «Horizon Europe» programme, Researchers’ Night is a major event for the dissemination of science that should not go unmentioned, and which includes no shortage of chemistry-related topics. Since 2005, this event has been held simultaneously in almost 500 European cities (including many in Spain), always on the last Friday of September.
Of course, there are also a wide variety of toys and games used, including scientific objects (e.g., the drinking bird, fortune-telling fish, storm crystal, and artificial snow, etc.), card games, and adapted conventional games (Battleship, Snakes and Ladders, the Alphabet Game, Trivial Pursuit, word searches, and puzzles, etc.), as well as computer applications based on these games, among others (Solbes Matarredona et al., 2008). For decades, the most classic of these are home-based laboratory kits for children – boxes of materials for experiments – which, for safety reasons, now contain increasingly simple reagents (Figure 7). Escape games are also highly appreciated by young audiences and are carried out both for commercial purposes and in schools (Tajuelo & Pinto, 2021).

Figure 4. Cover and title page of The golden book of chemistry experiments (1960) by Robert Brent. Shortly after its publication, this work was banned in the United States because of the dangerous nature of some of its experiments. / Internet Archive
As far as current radio and television programmes in Spain are concerned, some examples stand out in particular. Among the former are A hombros de gigantes (‘On the shoulders of giants’), hosted by the scientist Manuel Seara Valero (RNE), with Bernardo Herradón García as a chemistry expert, and Longitud de onda (‘Wavelength’; RNE Radio Clásica), in which Rafael García Molina presents aspects of physics and chemistry related to classical music. As for television, the most important programme is Órbita Laika (‘Laika Orbit’), which has been broadcast by RTVE for several seasons and is directed by Eduardo Sáenz de Cabezón. Its aim is to combine entertainment with the explanation of scientific concepts, including chemistry in a section presented by the chemistry populariser Deborah García Bello.
Of course, science museums also have permanent, travelling, or temporary exhibitions. Sometimes they are hosted on websites, which multiplies the possibilities for their access. One example is the exhibition Sciences Pour Tous (‘Science for all’) 1850–1900, presented in 2017, which is still available online.2
Finally, although this is not a common method, some chemistry teachers at different stages of education also use some of these abovementioned resources in their classes from time to time. Their objectives are usually to discuss the properties of the matter and to introduce concepts, create a more relaxed atmosphere in the classroom, and motivate students.
Characteristics of recreational chemistry
Among the «strengths and opportunities» to be highlighted in the field of recreational chemistry are the visualisation and participation in practical experiences, which is not always easily accessible (e.g., because of the use of specific reagents, safety requirements and knowledge required for handling reagents, and complex instrumentation, etc.), visualisation of knowledge about new topics taught by experts, accessibility to all kinds of audiences, ability to learn about chemistry in a relaxed way, and motivation to deepen and broaden one’s knowledge.
In turn, the «weaknesses and threats» include the foreseeable passivity of participants, some possibility of risks and accidents (which is greater than in other fields of knowledge because of the reagents and equipment used for chemical demonstrations), difficulties in transporting said equipment and reagents, potential saturation of information about recreational science in general because of the boom in this type of activity in the media (television, social networks, YouTube, etc.), projection of a distorted image of science and its concepts (especially if the person leading the recreational activity is not an expert in chemistry), and the possibility that a clearly «recreational» atmosphere might dilute the educational aspects.
To make the most of strengths and opportunities and to avoid or mitigate, as far as possible, the weaknesses and threats, we must rely on the work of popularisers as professionals well trained in dissemination activities. They are usually teachers at different levels of education, researchers with a special interest in dissemination, university students with a taste for these activities, and professionals dedicated exclusively to dissemination.

Figure 5. Contemporary communicators of chemistry. On the left, the English science populariser, Martyn Poliakoff, popular for his light-hearted videos on the periodic table. On the right, Lebanese American professor Bassam Z. Shakhashiri, who has done a great deal of work in popularising recreational chemistry through his publications and by giving hundreds of lectures in schools, shopping centres, and even on television. / Periodic Tables Videos CC BY-SA 3.0/ Science History Institute CC BY-SA 3.0
It is usually an «extra» task for both educators and researchers, who find no other incentive – and this is no small thing – than liking dissemination and the opportunity to complement their teaching with other types of activities (e.g., by writing a popular science book or participating in a science fair, etc.). However, the current trend is to consider this as an additional activity in the work of these professionals, through awards for these tasks and their inclusion as part of the results of funded research projects, etc. In any case, and as already pointed out two decades ago (Pinto, 2003), outreach should be considered as one of the tasks of science teachers.
There are a number of recommendations for professionals running recreational chemistry activities, many of which have been suggested by Peter Childs (2014), another very relevant English populariser in the field of chemistry today. These include: choosing experiments that are appropriate to the knowledge, skills, and level of the audience, as well as to the topic being presented; using demonstrations to encourage reasoning, not just to provide an immediate answer to a particular fact; not presenting activities as mere «magic» but rather, promoting audience interaction and involvement and encouraging observations during the presentation. To this end, it is also important to give a prominent role to audience questioning. «What if» questions are often very effective in improving the outcome of demonstrations, as is suggesting different hypotheses and testing them experimentally. It is also often appropriate to stimulate thinking with discrepant events. Finally, the «fun» aspect, which promotes an eccentric image of science, should not be overemphasised.

Figure 6. Advertisement for a job offer for a «servant» to work as a caretaker and instructor of children (emphasising «recreational and curious chemistry»), published in El Diario de Madrid in 1837 (no. 672, p. 4.) / Biblioteca Nacional de España
While it is important to take appropriate safety precautions in any recreational science activity, the effort should be even greater when it comes to chemistry, especially when hands-on experiments are involved. Some considerations in this respect can be found in Cesa et al. (1998).
Obviously, in addition to all the recommendations found in the literature on the subject, the experience and spontaneity of the disseminator always plays an important role in successes. A cartoon published some time ago showed a magician with a flask in his hand. He poured the contents into another and waited for a reaction with a blank expression on his face. Finally, he smiled again and said: «However, the change is spectacular on the molecular level».
Recreational chemistry in science education and science popularisation
Apart from its playful components, recreational chemistry can (and should) be an important tool not only for popularisation, but also for science education, even in mainstream education. For example, a «magic trick» in Tissandier’s book (1880/1884) about the alleged passage of smoke through into a covered glass (Figure 3D) has recently been taken up by Professor Luis Moreno as a very interesting «learning situation» to promote inquiry-based learning (Moreno Martínez, 2022). The final conclusion is that, after discussion, students can identify a very specific chemical reaction going on inside the glass: NH3(g) + HCl(g) → NH4Cl(s), in which the solid particles of ammonium chloride produced form a colloidal smoke mixture with the air (instead of a fog, which would be liquid droplets dispersed in a gas).

Figure 7. Box and reagent bottles of popular Spanish chemistry sets from the early 1970s, Cheminova and Quimicefa. These games included reagents that are not accessible to the general public today – especially not to children – because of their potential danger. In the author’s personal collection. / Gabriel Pinto Cañón
Integrating everyday chemistry into the classroom is not easy. Among other things, there is a tendency for students to see it as a time to relax when it is discussed in this way in class, with them tending to focus more on form than substance. In addition, there is always some controversy about the use of such activities in formal education. In a paper questioning competence-based education, De Azcárraga recently paraphrased Miguel de Unamuno (using a phrase he first uttered in 1913!): «We seem afraid to teach children how hard, how demanding it is to work. This has led to having them learn by playing, which always ends up as playing at learning. And the master who is teaching them plays, plays at teaching. And neither is he, strictly speaking, teaching, nor are they, strictly speaking, learning anything worth-while» (De Azcárraga, 2021).
Conclusions
Recreational chemistry is an area of interest for the dissemination, learning, and appreciation of this science because it allows a wide range of audiences to approach its contents in a relaxed and friendly way. To this end, adequate training of the people in charge of the dissemination process is essential. Indeed, the implementation of everything related to popularisation is becoming increasingly professionalised, through fairs, publications, blogs, and the other means mentioned in this current work, but also through institutions such as the FECYT (Spanish Foundation for Science and Technology), museums, and science popularisation units in universities, to name but a few.
In addition, access to such activities has been greatly facilitated by information and communication technologies. Although this may also facilitate the spread of misconceptions, it is definitely an open door that allows fun experiences, making it possible to approach a science, chemistry, whose concepts are not otherwise always easy to grasp.
It is important that the festive and playful nature does not overshadow the intended message (in this case, knowledge of chemistry and scientific reasoning). One could paraphrase Childs (2014) who suggests that demonstrations and activities should not be an end in and of themselves but rather, an open door to the field of chemistry, and so, these activities should be appropriately focused to account for this.
Finally, it should be noted that what is relaxed and fun for some may be boring or mediocre to others. In any case, what is important is that a well-established scientific task, such as the development of recreational chemistry, continues its course and opens up new channels as another element in facilitating the public appreciation and understanding of science.
References
Armstrong, E. V. (1938). Jane Marcet and her “Conversations on chemistry”. Journal of Chemical Education, 15(2), 53–57. https://doi.org/10.1021/ed015p53
Berman, M. (1972). The early years of the Royal Institution 1799-1810: A re-evaluation. Science Studies, 2(3), 205–240. https://doi.org/10.1177/030631277200200301
Cesa, I. G., Finester, D. C., Sigmann, S. B., & Wilhelm, M. R. (1998). Revising the division of chemical education safety guidelines for chemical demonstrations. Journal of Chemical Education, 95(4), 502–503. https://doi.org/10.1021/acs.jchemed.7b00802
Childs, P. (2014). Using demonstrations to stimulate inquiry and students’ thinking. Educació Química, 19, 48–57. https://doi.org/10.2436/20.2003.02.144
Crellin, J. K. (1979). Mrs. Marcet’s “Conversations on chemistry”. Journal of Chemical Education, 56(7), 459–460. https://doi.org/10.1021/ed056p459
De Azcárraga, J. A. (2021). La nueva legislación educativa: por qué no mejora la educación pública en España. Revista Española de Pedagogía, 80(281), 111–129. https://doi.org/10.22550/REP80-1-2022-08
García Belmar, A. (2020, 23 December). La ciencia en la esfera pública. Sabers en acció. https://sabersenaccio.iec.cat/es/la-ciencia-en-la-esfera-publica-es/
García-Molina, R. (2011). Ciencia recreativa: un recurso didáctico para enseñar deleitando. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 8 (Special issue), 370–392.
Hache-Bissette, F. (2017). Le partage des savoirs: Science populaire ou vulgarisations scientifique. In M. Netze (Ed.), Les sciences en bibliothéque, pp. 51–62. Éditions du Cercle de la Librairie.
Haran, B., & Poliakoff, M. (2011). The periodic table of videos. Science, 332(6033), 1046–1047. https://doi.org/10.1126/science.1196980
Kale, S. (2004). French salons, high society and political sociability from the old regime to the revolution of 1848. The Johnson Hopkins University Press.
Moreno Martínez, L. (2022). El valor educativo de la historia de la química para las aulas de secundaria. Anales de Química, 118(3), 163–171.
Parkes, S. (1819). The chemical catechism, with notes, illustrations and experiments. R. and A. Taylor.
Pinto, G. (2003). Divulgación de la química. Anales de Química, 99(4), 63–66.
Pinto, G., Castro Acuña, C. M., & Martínez Urreaga, J. (2006). Química al alcance de todos. Pearson Educación.
Puerto Sarmiento, F. J. (1994). La huella de Proust: El laboratorio de química del Museo de Historia Natural. Asclepio, 46(1), 197–220. https://doi.org/10.3989/asclepio.1994.v46.1.480
Richard. (1856). Le magicien des salons ou le diable couleur de rose. Recueil nouveau de physique amusante, de chimie récréative, de tours de cartes, magie blanche, etc., etc. Delarue.
Solbes Matarredona, J., Lozano Gutiérrez, Ó., & García Molina, R. (2008). Juegos, juguetes y pequeñas experiencias tecnocientíficos en la enseñanza aprendizaje de la física y química y la tecnología. Investigación en la Escuela, 65, 71–88. https://doi.org/10.12795/IE.2008.i65.07
Tajuelo, L., & Pinto, G. (2021). Un ejemplo de actividad de escape room sobre física y química en educación secundaria. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 18(2), 2205. https://doi.org/10.25267/Rev_Eureka_ensen_divulg_cienc.2021.v18.i2.2205
Tissandier G. (1884). Recreaciones científicas ó la física y la química sin aparatos ni laboratorio y solo por los juegos de la infancia con una exposición detallada de los principales aparatos que pueden constituir la casa o museo de un aficionado a las ciencias, seguida de algunas aplicaciones científicas a los usos de la vida doméstica, etc. (Spanish translation by E. Sánchez Pardo). Carlos Bailly-Bailliere. (Original work published in 1881).
Vélez de Paredes, E. (1860). Manual de química divertida, ó sea recreaciones químicas. Librería de Rosa y Bouret.
Acknowledgements
I would like to thank the Community of Madrid for their support through the Multi-Annual Agreement with the Universidad Politécnica de Madrid, within the framework of the «Excellence Programme for University Teaching Staff», framed within the V Regional Plan of Scientific Research and Technological Innovation (PRICIT).





