|
One of the privileges of working on this section of the journal Mètode is the chance to recount the surprising things we encounter and enjoy in the plant world. And that is what we are going to do here. From the sugars derived from photosynthesis, plants can make a wide variety of molecules, each of which has incredible properties. Saponins are just one of these types of molecule, made with chains of sugars to which other compounds are added. Well, if you take a stroll through the Botanic Garden of the University of Valencia you will come across extraordinary and very showy trees that have the word Saponin –meaning «soap» in Latin– in their scientific name. When a plant is so named, this means it has soap-related characteristics. Plants with the name Saponin can be found growing in Spain, like the Common Soapwort (Saponaria officinalis L.), and in other countries too, such as the American Soap bark tree, Quillaja saponaria Molina. The latter is also used to manufacture the foam in fire extinguishers. We can also find trees of the genus Sapindus (saponis-indicus), commonly known as «soapberries» or «soapnuts», native to Asia, Hawaii and America. In autumn, at the Botanic Garden you can see trees like the golden-rain tree from China, Koelreuteria paniculata Laxm., used by the Chinese for cleaning. It was Thomas Jefferson who introduced it to the United States in 1809 as an ornamental tree, which subsequently made its way to Europe. Since ancient times herbal soaps have been used for skin care and cosmetics. There are many plants, such as ivy, butcher’s broom, asparagus, alfalfa, soybeans and peanuts, containing the saponin molecule, which can be toxic in large quantities. To this sugar chain contained in the saponin, more or less water can be added, and this combination will produce foam. Every day, in one way or another, we all use soap for our hands, clothes, to clean the house… The seeds of these plants produce foam, and the property of soap relates to the foam it forms, which we like in abundance, though this also depends on the quality of water, and that is another story. The history of the bar of soap is long and fascinating. It all began with ash, which played the role of an alkaline, and with animal or vegetable fat. The Romans used goat fat and beech ashes. In Spain, olive oil was used and the soap from Castilla was famous for its softness. In many regions, plants and aromas were added to make a more sophisticated soap, like in Marseilles and Venice. Soap-making became a widespread practice and each added plants and scents, such as lavender, so-called Heno de Pravia. Later, the beech ash was replaced by soda ash or Salsola kali (prickly saltwort), a plant that grows in the sandy grasslands of Europe and is also used to make glass. Then, 1787 saw the discovery of how to obtain alkaline from common salt, which enabled the unlimited manufacture of soap. Thanks to this, soap became an everyday and necessary common good. In this issue, we propose soap making, while at the same time recycling. Maybe, this proposal will also help us understand chemistry better and give us an idea of what to do with everyday waste. Soap is the product of a chemical reaction, as if by magic, oil turns solid. To make this transformation come about, and permanently so, we must add a substance to be treated with care: an alkali (lye or sodium hydroxide). The chemical reaction called saponification is the result of combining an alkaline with a fatty acid of animal or plant origin, the most commonly used being the oil of palm, coconut, sunflower or olive, and shea butter. Also, saponins play a different role in cuisine, providing that nice foam on your beer! Re-using leftover oil from the kitchen to make soap would be really good for the environment, as every litre of oil tipped down the sink contaminates 50,000 litres of river-water. ■ SOAP-MAKING ACTIVITY
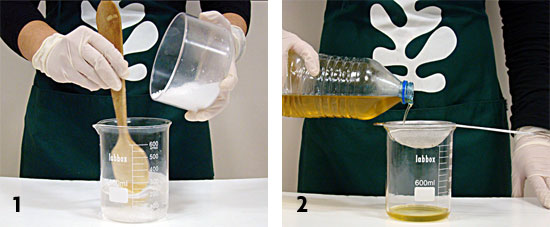
What to do: And, hey presto, you have soap! |
«Soap is the productof a chemical reaction, as if by magic, oil turns solid!»
Following these easy steps, with just a few materials, you can easily make practical bars of soap by recycling used oil, as shown in the pictures. |