Sex and design in our evolutionary cousins
The perception of beauty in nature
Taking an evolutionary approach to the question of beauty, we discuss the expression and perception of sexual beauty across the animal kingdom. Animals experience beauty in their brains, and animal brains are tuned to features of the environment most relevant to their survival. Over evolutionary time, sexually reproducing animals have exploited that tuning to maximize their attractiveness to the opposite sex, often leading to extreme courtship traits and behaviors. These are the traits of sexual beauty. Combining modern principles of neuroscience and neuroaesthetics with established principles of evolutionary biology, we aim to understand the biological basis and evolution of beauty in all animals, including ourselves.
Keywords: beauty, neuroaesthetics, perceptual fluency, sensory bias, sexual selection.
Beauty is said to be in the eye of the beholder, but beyond the popular adage, a clear definition eludes us. Indeed, philosophers and scientists have struggled to define beauty throughout history, and we continue to seek its meaning. Given the lack of a robust or consensus definition, you’d think we haven’t made much progress, but that’s not quite true. Early Greek philosophers proposed that beauty was an objective quality, an inherent property of beautiful things. If a thing was ordered and organized to perfectly suit its function, it was beautiful, by definition, no matter who perceived it. Today, most scientists disagree. They find instead that beauty is not objective but subjective, an emergent property of our mind as it interacts with its environment. We respond to objects or sounds in the world, yes, but the experience of beauty is a process that takes place in our heads. When we’re talking about beauty, we’re talking about brains. Beauty may sometimes be in the eye of the beholder, but it is always in its brain.
The modern field of neuroaesthetics developed around this central role of the brain. Neuroaesthetics began as the study of human responses to art, primarily visual art, but the field has grown to include not only different forms of art, like music and poetry, but also more biologically relevant stimuli, like landscapes and faces. The field has also expanded through progress in neuroscience, incorporating increasingly sophisticated metrics of neural activity like electroencephalography (EEG), positron emission tomography (PET), and functional magnetic resonance imaging (fMRI). Neuroaesthetics is therefore a quintessentially interdisciplinary field of study, linking scholars across the humanities, neuroscience, psychology, computer science, and evolutionary biology. It is from the perspective of evolutionary biology that we consider the question of beauty.
As humans, we find beauty all around us. As evolutionary biologists, we ask where this «taste for the beautiful» originated, and as behavioral ecologists, we ask to what degree this taste is manifest in other animals, and what kinds of things animals would find beautiful. For humans, the most biologically relevant beautiful thing is, arguably, another human face, especially those to whom we are sexually attracted. Thus, a likely candidate for beauty in animals is the features they use to attract members of the opposite sex (Prum, 2017; Ryan, 2018). These so-called «secondary sexual traits» are ubiquitous in nature, and in some animals, like the resplendent quetzal (Pharomachrus mocinno), they’ve gone hog wild (Figure 1).

Sexual selection
Secondary sexual traits have been associated with the science of beauty at least since Darwin, who said that «the senses of man and of the lower animals seem to be so constituted that brilliant colours and certain forms, as well as harmonious and rhythmical sounds, give pleasure and are called beautiful; but why this should be so we know not». Substituting «people» for «man», and «other» for «lower», his question still resonates as we investigate the origin and biological basis of beauty throughout the animal kingdom. Indeed, Darwin’s focus on pleasure placed appropriate emphasis on the brain as the most important sex organ, and it forms the foundation of his theory of sexual selection (Darwin, 1871). Even though Victorian England was loathe to admit it (Richards, 2017), Darwin’s theory of sexual selection probably had it right all along by focusing on the power of the mind to generate beauty in nature.
Sexual selection is responsible for generating much of the beauty in the animal kingdom. Traits that evolve by sexual selection enhance an animal’s ability to acquire mates; some of these traits are armaments and some are ornaments. Armaments are weapons that promote battles to obtain mates, while ornaments evolved to charm members of the opposite sex – these are the traits of sexual beauty (Rosenthal, 2017). Darwin concerned himself with the most typical mating systems: males – these are the «courters» – use ornaments to compete for the attention of females, and females – the «choosers» – decide who mates (Darwin, 1871). But since Darwin’s time, substantial variation on this theme has been uncovered, including traits that function as both ornaments and armaments, species in which females are courters and males are choosers, and in many mating systems, especially in humans, in which both sexes choose (Rosenthal & Ryan, 2022).
Besides being beautiful, sexual ornaments are usually costly to produce and maintain; in many cases, they attract not only females but predators. Darwin proposed that these traits could still evolve if the benefit of mate attraction outweighed the cost of lower survivorship (Darwin, 1871). Much research in behavioral ecology has asked what benefits choosers gain from choosing beautiful traits. But here, we are concerned with what makes these traits beautiful in the first place. Darwin gave us some hints: «When male animals utter sounds in order to please females, they would naturally employ those which are sweet to the ears of the species» (Darwin, 1872). Since beauty is in the brain of the beholder, much of the recent research in mate choice has explored the neural, perceptual, and cognitive architecture underlying a taste for the beautiful.
The neural and social basis of beauty
Brains might be the most important sex organ, but they have other things on their minds. The neural architecture of a brain is shaped not just by sex but by the highly dimensional ecological niche in which it evolved. The Earth is comprised of an immeasurable number of distinct ecological niches to which brains can adapt – even within the same niche, different adaptations can solve the same problem – resulting in a commensurate diversity of neural architectures. An animal that is hunted in the daytime, for example, might devote significant neural resources to vision in full-spectrum light to detect predators. Alternatively, that animal might invest more of its neural resources in the proprioceptive and motor skills needed to outrun predators. These neural specializations create what von Uexküll called the animal’s Umwelt – the «inner world» through which an animal perceives and acts on its environment (von Uexküll, 2014). A diversity of niches and adaptations has led to striking diversity in the inner worlds of animals, and these neuroecological adaptations create biases – from sensation to perception, cognition to decision – that dictate whether an animal finds something attractive or beautiful.
Perceptions of beauty are formed in the brain, but the essence of beauty must first be transduced into neural responses by sensory organs. These are the portals to the brain through which sounds, sights, smells, and other sensations must pass, through ears, eyes, nares and other organs, in order to reach the brain. But these stimuli do not get a free pass. The sensory organs are gatekeepers, allowing only a subset of stimuli to pass through. All sensory organs are tuned; that is, they are more sensitive to some stimuli than to others. For example, we cannot see the ultraviolets in bird plumage, nor hear the ultrasonic echolocation calls of bats, and we can only detect a subset of the bouquet of odors perceived by dogs. Naturally, animals should evolve courtship signals to which the animal’s sensory systems are already sensitive, and when this happens we call it sensory exploitation (Ryan, 2018).

Figure 2. A calling male túngara frog (Engystomops pustulosus). Animals develop courtship strategies based on the stimuli to which they are sensitive. The male túngara frog and its closest relative are the only ones to use the basilar papilla for communication, and the pitch of their call evolved to coincide with the pre-existing tuning biases in the basilar papilla. This extra sensory stimulation results in females finding those calls much more sexually beautiful. / Photo: Ryan Taylor
For example, frogs have two different ear organs that process sound, called the amphibian papilla and the basilar papilla, and these organs are tuned to distinct pitches. The túngara frog Engystomops (= Physalaemus) pustulosus (Figure 2) and closely related species all produce whine-like calls that stimulate the amphibian papilla, but only the túngara frog and its closest relative (Engystomops petersi) add syllables, called chucks, that match the tuning of the basilar papilla. These chucks increase the sexual attractiveness of the whine five-fold! Even though only these two species use the basilar papilla for communication, all of the túngara frog’s close relatives have basilar papillae with the same tuning. Thus, the pitch of the chuck evolved to match the pre-existing tuning biases in the basilar papilla (Wilczynski et al., 2001). This extra sensory stimulation results in females finding those calls much more sexually beautiful.
Evidence for analogous patterns in visual communication abounds (Cummings & Endler, 2018). In Lake Victoria, for example, the color of the light environment varies with depth. Cichlid fish that live at different depths in this lake, therefore, have different color sensitivities because the colors to which their eyes are most sensitive are tuned to their native light environment. Following suit, the color of male courtship displays has evolved to match the tuning of the eye. In a bizarre example involving odors, male orchid bees try to mate with deceptive orchids, which mimic the odor of female bees to attract males to their flowers who then inadvertently act as pollinators (discussed in Ryan, 2018).

Figure 3. A sample of variation in coloration of male guppies from Trinidad (Poecilia reticulata). This fish is known not only for an assortment of colors in males but also for the variety of patterns in which these colors occur. Guppies might be capitalizing on a perceptual bias for high contrast, which can be achieved by a variety of patterns. / Photo: Anne Houde
Once these stimuli, or more precisely the neural responses they elicit, reach the brain, they are subject to higher-order processing that results in the animal’s percepts, or perceptions, of beauty. And when it comes to the perception of beauty, guppies (Poecilia reticulata) are the stars (Figure 3). These colorful little fish are the lab rat of sexual selection research, known not only for an assortment of bright colors in males but also for the variety of patterns in which these colors occur. As in art, it is not merely colors that give pleasure but the patterns in which those colors are arranged, and patterns are processed as percepts. One explanation for variation in the color patterns of male guppies could be the variation of color sensitivity in the eyes of these fish. Different individuals might perceive different colors as brighter, for example (Houde, 1997). Or, guppies might be capitalizing on a perceptual bias for high contrast, which can be achieved by a variety of patterns. Sibeaux and colleagues found that female guppies are most attracted to males with the highest variation in contrast across their bodies (Sibeaux et al., 2019). This variation in contrast could prevent sensory adaptation, a kind of neurological boredom, as females scan the male body.
Marilyn Monroe is quoted as saying «it is better to be absolutely ridiculous than absolutely boring». Getting bored is the death knell for sexual beauty, and patterns are also important in the context of habituation, the behavioral version of boredom. This was demonstrated quite clearly in a simple but elegant experiment in (who else) the guppy. Researchers raised two lines of guppies in the lab. The males in the two lines did not differ conspicuously in the type or the amount of colors they expressed, but they did differ in the arrangement of the colors, i.e., their patterns. Sexual responsiveness in females waned when presented males in their own line but was restored when they were exposed to males in the other line who displayed a novel pattern (Daniel et al., 2019).
Fighting against habituation is also thought to explain the evolution of song repertoires in birds (Hartshorne, 1973). In zebra finches, both brain activity and sexual behavior increase when they are exposed to a novel versus a familiar song note (Dong & Clayton, 2009). As with the relationship of colors and patterns, the patterns of sound are critical to the beauty of the bird’s song – this is true both for the birds and for us. Bilger and colleagues showed that natural patterns of notes in the songs that birds actually sing are more beautiful to us than those same notes in a random order (Bilger et al., 2021). These studies suggest that birds and humans share perceptions of the attractiveness of higher-order patterns of song notes. Of course, Darwin saw this coming. Especially when it came to bird plumage and bird song, Darwin thought we and the birds might share the same aesthetic preferences: «On the whole, birds appear to be the most aesthetic of all animals, excepting of course man, and they have nearly the same taste for the beautiful as we have» (Darwin, 1871). These influences of habituation further emphasize that beauty is not an inherent quality of the object alone but rather how that object is perceived.
It is now becoming clear that perceptions of sexual beauty can be malleable; for example, the behavior of others can change our perceptions. In what has become a classic experiment, Dugatkin and Godin (1992) demonstrated what we now call «mate choice copying». In a controlled laboratory experiment, a female guppy is allowed to choose between two males. She chooses one, the preferred male. Then she witnesses the unpreferred male courting a model female. The model is removed and the female now switches her preference to the previously unpreferred male. We now know that mate choice copying is rampant in the animal kingdom, including us.
For example, a man pictured with an attractive woman is rated as more attractive by women subjects compared to the picture of the same male by himself. But why? Recently, Street and colleagues asked subjects to rate the attractiveness of a photo on a sliding scale. They were then given feedback as to how other subjects rated this same photo, and after that given an opportunity to change their rating. On average, the subjects shifted their rating about 13 % towards the majority opinion. The researchers conducted the same study where the subjects rated the attractiveness of hands, and of works of art. Amazingly, the 13 % shift of the rating toward the majority opinion was exhibited in both of these experiments. The conclusion is that, yes, mate choice copying does occur in humans, but it might be a domain-general manifestation of social facilitation (Street et al., 2018). Taken together, these studies show that animal brains have biases, from sensation to perception to social facilitation. Our brains are shaped by our evolutionary history and can be exploited by potential mates to increase their beauty.
Wanting and liking: beauty and the reward system
An exciting new line of research in the study of beauty considers the role of the brain’s reward system. Our reward systems are reasonably well-defined regions of the brain that make us «like» something (the reward) and also «want» it. These distinct psychological mechanisms of liking and wanting were demonstrated clearly by Berridge and Robinson in a study of laboratory rats, the actual lab rats of comparative psychology (Berridge & Robinson, 1998). Rats like sugar. But if they lack dopamine (i.e., if dopaminergic neurons are destroyed by administering a neurotoxin), they no longer want it, i.e., they no longer work to obtain it, even though they clearly like it when a researcher gives it to them. The liking-wanting framework is potentially highly relevant for understanding beauty, since, arguably, the only consensus view of beauty is that it involves some kind of pleasure. The ability to measure pleasure as its own physiological process is, therefore, critical for understanding responses to sexual stimuli. Animals clearly «want» sex, and they probably «like» it too. A question worth asking then is whether secondary sexual traits, which constitute so much of beauty in nature, elicit pleasure, or liking, in the animals for whom they are intended.
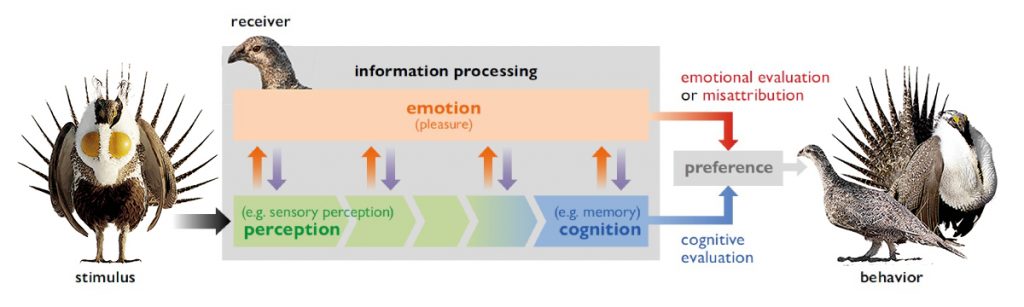
Figure 4. Environmental stimuli (e.g., a male sage grouse courtship display) are processed by perceptual and then cognitive neurons of a receiver (e.g., a female sage grouse). When information processing is effective or efficient (e.g., when a display mimics the spatial statistics of natural scenes; orange arrows), it generates pleasure. This pleasure could contribute to a fast and positive emotional evaluation (red arrow) and could further regulate the process of information gathering (e.g., motivate further attention; violet arrows). The pleasure response is misattributed to the stimulus, rather than to the information processing, thus biasing appetitive behavior towards the stimulus (red arrow). / Source: Adapted from Renoult & Mendelson (2019). / Photographs by Alan Krakauer and Tom Koerner
Plenty of evidence indicates that secondary sexual traits trigger the reward circuitry of animals (Lynch & Ryan, 2020). For example, male mating songs trigger the mesolimbic reward system in animals as different as white-throated sparrows (Maney, 2013) and túngara frogs (Hoke et al., 2010). These and other studies make it clear that secondary sexual traits trigger wanting, or incentive salience. There is also evidence that secondary sexual traits can elicit pleasure (liking), independent of their ability to elicit desire (wanting). Using two different behavioral responses, Dai and colleagues found that people judge the «likability» of attractive human faces separately from their «desirability» (Dai et al., 2010). The exciting path forward, then, is to develop studies like these for nonhuman animals. Such studies can tell us whether secondary sexual traits trigger liking as well as wanting, as we expect if animals find these traits as beautiful as we do.
Theoretical evidence suggests they will. Experimental psychologists use the term perceptual fluency to describe the positive emotions that are elicited by information that is easily processed by the brain. For example, people generally like symmetrical images, and images with continuous lines or surfaces, which are easy for the brain to encode. At the same time, researchers in empirical aesthetics have shown that images with the spatial statistics of natural scenes, i.e., the patterns and shapes found in nature, are both easily processed by the brain and positively judged (liked) by people. Even the characters in our written languages mimic the statistics of natural scenes (Changizi et al., 2006)! Our brains evolved in nature, so they are tuned to the patterns most important to our survival and reproduction; it is no wonder that we can most easily process natural patterns. And when information processing is easy, we experience pleasure: we «like» it. As humans, we are literally drawn to images that are «easy on the eyes».

Figure 5. Variation in colors and patterns in some North American darters. One of the open questions remaining about the beauty of animal ornaments is to what extent a processing bias can explain it. In the case of humans, evidence supports that easily processed faces are considered more attractive. / Photo: Samuel Hulse
Might animals other than humans share this bias for the patterns of nature, and could this explain the beauty of secondary sexual traits? Renoult and Mendelson (2019) think so, at least in part. They refer to a preference for easily processed patterns as a «processing bias», adding to the list of biases described above for colors, sounds, and patterns. A processing bias might be especially relevant for perceptions of beauty, as it explicitly identifies a role for pleasure, in the efficient processing of information (Figure 4). A processing bias could be exploited by courters to increase their attractiveness, for example, if secondary sexual traits mimic the spatial statistics of natural scenes. Evidence in support of that hypothesis includes studies of human faces, which show that easily processed faces are considered more attractive (Holzleitner et al., 2019; Renoult et al., 2016), and a study of colorful fishes (Figure 5), which showed that beautiful male breeding patterns mimic the spatial statistics of the habitats in which they evolved (Hulse et al., 2020). To what extent a processing bias explains the beauty of animal ornaments, as it appears to explain some of the beauty of human designs, remains another exciting line of research.
Concluding remarks: understanding the meaning of beauty
When it comes to beauty, we have a lot to learn from our evolutionary cousins. In life as in art, beautiful things are designed to evoke pleasure. Whether designed by a female quetzal over evolutionary time or by Georgia O’Keeffe on a hot summer day, the brains of animals are both the creators and the beneficiaries of beauty in nature. Surely some of the beauty in nature is invisible to us, evolved only for the brains of other beholders. Ecological diversity generates neurological diversity, which in turn generates a diversity of beauty specialized to the niche in which it evolved. But we’re lucky to still share many of the perceptual and cognitive biases of our evolutionary kin, because their beauty is our beauty too. We may not have come as far as we’d like in our search for the meaning of beauty, but applying new insights from the field of neuroaesthetics to established principles of evolutionary biology holds much promise.
References
Berridge, K. C., & Robinson, T. E. (1998). What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 28(3), 309–369. https://doi.org/10.1016/S0165-0173(98)00019-8
Bilger, H. T., Vertosick, E., Vickers, A., Kaczmarek, K., & Prum, R. O. (2021). Higher-order musical temporal structure in bird song. Frontiers in Psychology, 12, 1–11. https://doi.org/10.3389/fpsyg.2021.629456
Changizi, M. A., Zhang, Q., Ye, H., & Shimojo, S. (2006). The structures of letters and symbols throughout human history are selected to match those found in objects in natural scenes. The American Naturalist, 167(5), E117–E139. https://doi.org/10.1086/502806
Cummings, M. E., & Endler, J. A. (2018). 25 years of sensory drive: The evidence and its watery bias. Current Zoology, 64(4), 471–484. https://doi.org/10.1093/cz/zoy043
Dai, X., Brendl, C. M., & Ariely, D. (2010). Wanting, liking, and preference construction. Emotion, 10(3), 324–334. https://doi.org/10.1037/a0017987
Daniel, M. J., Koffinas, L., & Hughes, K. A. (2019). Habituation underpins preference for mates with novel phenotypes in the guppy. Proceedings of the Royal Society B: Biological Sciences, 286, 20190435. https://doi.org/10.1098/rspb.2019.0435
Darwin, C. (1871). The descent of man and selection in relation to sex. John Murray.
Darwin, C. (1872). The expression of emotions in man and animals. John Murray.
Dong, S., & Clayton, D. F. (2009). Habituation in songbirds. Neurobiology of Learning and Memory, 92(2), 183–188. https://doi.org/10.1016/j.nlm.2008.09.009
Dugatkin, L. A., & Godin, J.-G. J. (1992). Reversal of female mate choice by copying in the guppy (Poecilia reticulata). Proceedings of the Royal Society B: Biological Sciences, 249, 179–184. https://doi.org/10.1098/rspb.1992.0101
Hartshorne, C. (1973). Born to sing. Indiana University Press.
Hoke, K. L., Ryan, M. J., & Wilczynski, W. (2010). Sexually dimorphic sensory gating drives behavioral differences in túngara frogs. Journal of Experimental Biology, 213(20), 3463–3472. https://doi.org/10.1242/jeb.043992
Holzleitner, I. J., Lee, A. J., Hahn, A. C., Kandrik, M., Bovet, J., Renoult, J. P., Simmons, D., Garrod, O., DeBruine, L. M., & Jones, B. C. (2019). Comparing theory-driven and data-driven attractiveness models using images of real women’s faces. Journal of Experimental Psychology. Human Perception and Performance, 45(12), 1589–1595. https://doi.org/10.1037/xhp0000685
Houde, A. E. (1997). Sex, color, and mate choice in guppies. Princeton University Press.
Hulse, S. V., Renoult, J. P., & Mendelson, T. C. (2020). Sexual signaling pattern correlates with habitat pattern in visually ornamented fishes. Nature Communications, 11(1), 1–8. https://doi.org/10.1038/s41467-020-16389-0
Lynch, K. S., & Ryan, M. J. (2020). Understanding the role of incentive salience in sexual decision-making. Integrative and Comparative Biology, 60(3), 712–721. https://doi.org/10.1093/icb/icaa054
Maney, D. L. (2013). The incentive salience of courtship vocalizations: Hormone-mediated 'wanting' in the auditory system. Hearing Research, 305(1), 19–30. https://doi.org/10.1016/j.heares.2013.04.011
Prum, R. O. (2017). The evolution of beauty: How Darwin’s forgotten theory of mate choice shapes the animal world-and us. Doubleday.
Renoult, J. P., Bovet, J., & Raymond, M. (2016). Beauty is in the efficient coding of the beholder. Royal Society Open Science, 3(3), 160027. https://doi.org/10.1098/rsos.160027
Renoult, J. P., & Mendelson, T. C. (2019). Processing bias: Extending sensory drive to include efficacy and efficiency in information processing. Proceedings of the Royal Society B: Biological Sciences, 286(1900), 20190165. https://doi.org/10.1098/rspb.2019.0165
Richards, E. (2017). Darwin and the making of sexual selection. University of Chicago Press.
Rosenthal, G. G. (2017). Mate choice: The evolution of sexual decision making from microbes to humans. Princeton University Press.
Rosenthal, G. G., & Ryan, M. J. (2022). Sexual selection and the ascent of women: Mate choice research since Darwin. Science, 375, eabi6308. https://doi.org/10.1126/science.abi6308
Ryan, M. J. (2018). A taste for the beautiful. Princeton University Press.
Sibeaux, A., Cole, G. L., & Endler, J. A. (2019). The relative importance of local and global visual contrast in mate choice. Animal Behaviour, 154, 143–159. https://doi.org/10.1016/j.anbehav.2019.06.020
Street, S. E., Morgan, T. J. H., Thornton, A., Brown, G. R., Laland, K. N., & Cross, C. P. (2018). Human mate-choice copying is domain-general social learning. Scientific Reports, 8(1), 1715. https://doi.org/10.1038/s41598-018-19770-8
Von Uexküll, J. (2014). Umwelt und Innenwelt der Tiere. Springer-Verlag.
Wilczynski, W., Rand, A. S., & Ryan, M. J. (2001). Evolution of calls and auditory tuning in the Physalaemus pustulosus species group. Brain, Behavior and Evolution, 58(3), 137–151. https://doi.org/10.1159/000047268