Miniature Universe: Challenges Facing Molecular Nanoscience.
The molecular field of Nanoscience is an area as yet little explored in Nanoscience. This may be because, compared to simpler atom-based nano-objects, the larger structural and electronic complexity of molecules makes them more difficult to study at nanoscale with the instrumental techniques available today. Nonetheless, it is in this molecular field where molecular chemists, biologists, physicists and engineers working in Nanosciences may have the best opportunities to interact. Areas like supramolecular chemistry, molecular electronics and molecular magnetism are expected to converge in this area. Here, the author gives the examples of bio-magnetic nanomaterials and multifunctional magnetic materials to illustrate the opportunities provided by this emergent area in chemistry, physics and materials science.
The chemist, as an architect of matter, can design and create increasingly complex molecules, which exhibit useful physical, chemical or biological properties. For example, molecules that can perform electronic functions, which may be instrumental to developing molecule-based electronic components that would provide an alternative to silicon-based electronics, thus allowing the manufacture of much smaller, more efficient and faster devices than those used today. This trend towards miniaturization is part of a multidisciplinary area called nanoscience. Increasingly sensitive techniques are becoming available with which to view, manipulate and measure the properties of atoms and molecules individually. Furthermore, in the nanoscopic world matter behaves differently to how it does in our macroscopic world. All in all, these prospects are a source of interest, making this new research area attractive to physics, chemistry, biology, medicine and engineering, alike. While for many, nanotechnology represents the scientific and technological revolution of the 21st century.
«Increasingly sensitive techniques are becoming available with which to view, manipulate and measure the properties of atoms and molecules individually»
The molecular facet of this field is poorly developed, probably because molecules are more complex objects than atoms, making them difficult to study at the nanoscale with the instrumental techniques available. However, it is precisely in this region of the nanocosmos where chemists and biologists can find the best opportunities to interact with physicists. In fact, with a view to creating and manipulating new functional molecular and supramolecular systems, molecular nanoscience seeks to build upon the knowledge of synthetic chemists and their understanding of molecular recognition and self-organization processes, which occur in biological systems. In turn, these new nanostructures represent a challenge to the instrumental and theoretical capabilities currently available to physics, while at the same time, they provide an opportunity to develop new applications in emerging areas such as molecular electronics, molecular spintronics and biomedicine. Scientists working in molecular nanoscience are focussing their attention, in order of increasing size, on functional molecules, followed by nanoparticles and other nano-objects of finite size such as carbon nanotubes, and finally infinite sized molecular nanostructures formed by self-organized molecular units in one, two or three dimensions (chains, monolayers, multilayers and, finally, crystals). This article gives two examples of molecular magnetism to illustrate how problems in molecular nanoscience are currently being addressed, and the most important challenges and opportunities within this area of science.
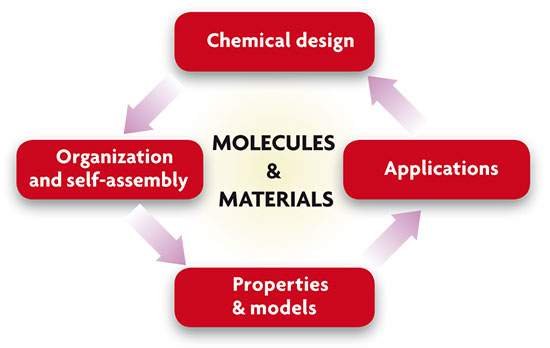
Research into molecular nanoscience encompasses four stages, as shown in this figure. The first step is to design functional molecules and other molecular nanomaterials; followed by the organization and self-assembly of these molecules to create molecular nanostructures. The next step is to study the properties of these systems, and finally, develop applications.
Biomolecules as nanoreactors
Ferritin is the protein responsible for storing iron in animals and plants. This biomolecule is some 12 nm in diameter and harbours a nanoparticle formed by, at most, 4500 iron atoms. This biomolecule is of interest in nanochemistry and the iron oxyhydroxide nanoparticle can be extracted via chemical treatment. Thus apoferritin is obtained, a porous protein that chemists can use as a biomolecular nano-reactor in chemistry. For example, a reduction in different metal ions in the interior of the apoferritin leads to the preparation of a whole series of metal nanoparticles (Co, Ni, Pd…). Thus, we can obtain nanoparticles of a specific size, which are also water soluble and, unlike the bare metallic nanoparticles, do not form aggregates.
«In the nanoscopic world matter behaves differently to how it does in our macroscopic world»
The above features can be useful to address unanswered questions in nanophysics such as, for example, the magnetism of palladium. In the macroscopic state, this metal is not magnetic, but theoretically it is assumed that ferromagnetism can occur when in the form of thin films or nanoparticles. The experimental problem is that it is not easy to prepare nanoparticles of a specific size and which, moreover, do not aggregate in the solid state. Notwithstanding, isolated palladium nanoparticles of a controlled size have been obtained within apoferritin, and theorists have proven right: palladium is ferromagnetic at room temperature when it is in the form of nanoparticles.
Once we have shown that we can perform chemistry within these biomolecules, the challenge facing nanoscience is how to organize these nano-objects on surfaces in a controlled way and with nanometric precision. In the short term, this would allow us to design magnetic nanostructures. In the longer term, such nanostructures may be of interest in nanoelectronics and nanomagnetism with a view to developing high-density magnetic memory, based on magnetic nanoparticles. One way to achieve this goal is to take advantage, on one hand, of the nanometric precision provided by local oxidation nanolithography, which uses atomic force microscopy to create silicon oxide dots of similar dimensions to ferritin, on a silicon surface. On the other hand, it takes advantage of the attractive and repulsive electrostatic interactions established between the ferritin molecule and the surface, to place the ferritin molecules exclusively on the spots of silicon oxide. Therefore, this procedure allows one to selectively place the ferritin molecule on silicon oxide nanostructures manufactured lithographically. The role of the protein is to transport the nanoparticle from the solution to the surface. The protein can be removed by physical processes (heating the sample in the presence of oxygen). Since we can synthesize a variety of nanoparticles (metals, metal oxides, metal chalcogenides, bimetallic cyanides…) in the interior of the protein, this procedure will allow the formation of a wide assortment of functional nanoparticles.

«Physics, chemistry, biology, medicine and engineering, all take an interest in nanoscience, which for many represents the scientific and technological revolution of the 21st century»
These magnetic biomolecules can also have medical applications. Taking advantage of the biocompatibility of apoferritin and its ability to harbour magnetic species, it is possible to design new contrast agents for Magnetic Resonance Imaging (MRI) based on low-toxicity and high-sensitivity ferritin derivatives. The limited sensitivity of the images obtained by MRI makes it necessary to search for increasingly sensitive contrast agents. This requires concentrating a large number of magnetic centres in a small space. Another essential condition is that the agent should not prove toxic during the time needed for diagnosis. The apoferritin molecule can trap compounds or particles with many magnetic centres, as well as being biocompatible. In particular, it can capture a manganese oxyhydroxide particle, which can be reduced to give a large number of MnII paramagnetic species, which significantly improves the magnetic relaxation properties compared to an alternative approach, in which gadolinium complexes are stored inside ferritin.
Design of multifunctional materials
Multifunctionality is a widespread trend in current materials science. In this context, molecule-based materials are attracting enormous attention because, in addition to exhibiting the most interesting technological properties traditionally associated with the atom-based inorganic materials (ferromagnetism, electrical conductivity and superconductivity, ferroelectricity, non-linear optics, etc.), molecular chemistry is highly versatile in terms of materials design. Thus, by choosing suitable molecular building blocks, supramolecular structures can be built, through a process of self-assembly, combining two properties that would be difficult or impossible to mix in a conventional inorganic solid. This possibility opens up new horizons for potential application in molecular electronics and spintronics.
An appealing approach to obtaining multifunctional materials is to construct solid hybrids formed by two molecular networks in such as way that each confers a property to the resulting material. For the molecular chemist, the search for multi-functionality involves the challenge of developing appropriate strategies to selectively synthesize hybrid materials having high chemical and structural complexity. Such materials can provide physicists the unique opportunity to observe new physical phenomena due to unusual coupling or association of properties.
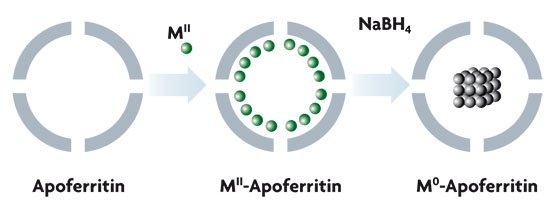
Figure of a chemical process inside ferritin, obtaining soluble metallic nanoparticles. The iron oxyhydroxide nanoparticle inside the ferritin is removed chemically, leading to apoferritin acquisition. A solution of metallic ions is placed inside the cavity, followed by reduction via treatment with an appropriate reducing agent, leading to the formation of a metallic nanoparticle in the apoferritin cavity. The size of the metallic nanoparticle is controlled by the size of the apoferritin cavity. Moreover, as these nanoparticles are apoferritin coated, they do not aggregate and are water soluble. Thus, palladium nanoparticles have been obtained and ascertained to exhibit ferromagnetic behaviour, according to the theoretical predictions.
«A challenge posed by this nwe type of material concerns the possible coexistence of ferromagnetism and superconductivity WITHin the same material, a subject of great interest in physics»
As a paradigmatic example of this hybrid approach, we can mention materials in which electric and magnetic properties coexist. To design molecular magnetic conductors we need to combine magnetic coordination complexes with organic conducting layers based on TTF-type p-electronic donor molecules (tetrathiafulvalene), which are stacked in the solid state, giving rise to energy bands with delocalized electrons. This approach has yielded materials that combine conductivity with the paramagnetic characteristic of magnetic molecules. However, it has not been possible to design ferromagnetic materials using this approach. To achieve ferromagnetic behaviour, an anionic polymer network has been used as an inorganic component rather than using a discrete species. The first milestone, in this sense, uses bimetallic oxalate complexes with a layered structure as the magnetic component.
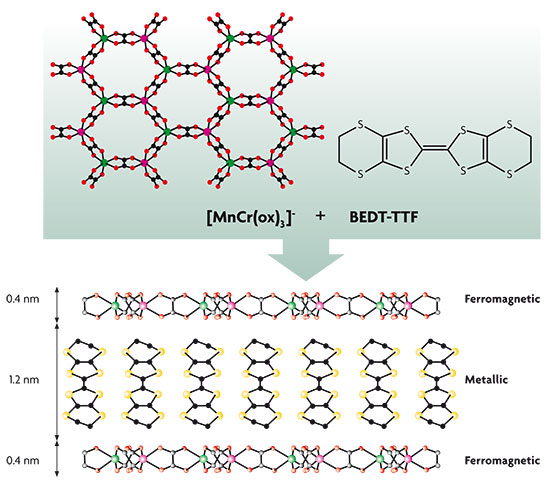
Design of a multifunctional ferromagnetic and metallic material. The elements assembled are negatively charged inorganic layers, formed by oxalate anions and manganese and chromium ions [MnCr(ox)3]–, and positively charged organic molecules of bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF). The inorganic layer means that, from the viewpoint of their magnetic properties, the compound obtained behaves like a ferromagnetic material. Meanwhile, the organic molecules are stacked to form layers sandwiched between the inorganic layers. This particular stacking means that, in terms of its electrical properties, the compound obtained behaves like a metal.
A challenge posed by these new types of materials concerns the possible coexistence of ferromagnetism and superconductivity in the same material, which is a topic of great interest in physics. Again, and like ferromagnetism, superconductivity requires exact packing of organic molecules in the solid state, a parameter which falls outside the realms of chemical control. In any event, as chemists we can go one step further and use a superconducting layer, as in the previous example we used a ferromagnetic layer. This strategy has proven successful to prepare a laminated solid, formed by alternating nanometrically thin layers of a ferromagnetic bimetallic oxide and of superconducting sulphur tantalum. This bottom-up method of nanoscience, based on the assembly of molecular or macromolecular building blocks found in dissolution, contrasts with the approach used in physics to produce superconducting and ferromagnetic heterostructures, based on growing these layers by successive evaporation of two metals. This difference leads to an entirely new situation from the viewpoint of physics. For example, the magnetic component is also a conductor in the physical heterostructures, whereas in the chemical heterostructures the magnetic component is an insulator or semiconductor. Again, this difference is expected to lead to new physical phenomena that prompt the development of new theories in this field.
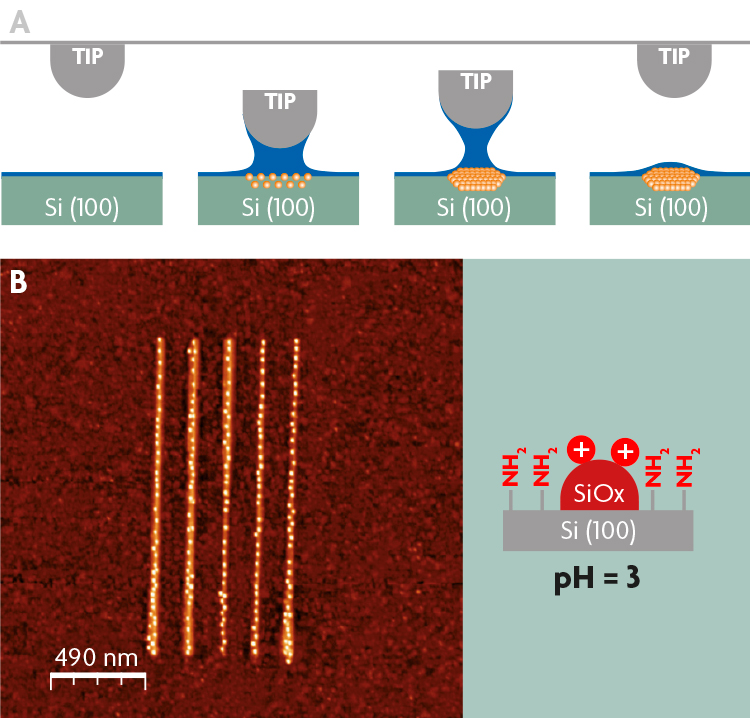
Organization of ferritin on a silicon surface, resulting from the electrostatic interactions established between the ferritin, the silicon surface and silicon oxide strips drawn by Local Oxidation Nanolithography (LON). The LON technique is shown in Figure A. It is a tip-based nanofabrication method via oxidation reaction that is locally confined to the vicinity of the material that is very close to, or in contact with, the tip of an atomic force microscope. Working conditions mean the material is covered by a thin layer of water. When the tip of the microscope approaches, and having established suitable voltage pulse differences between the tip and material, water bridges form between them and, at the same time, surface oxidation of the material begins in that area. Thus reproducible dots of silicon oxide, smaller than 10 nm, can be produced on a silicon surface, as shown in Figure B.
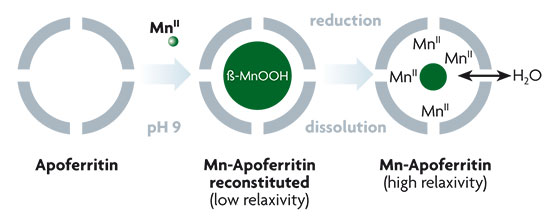
Chemistry inside ferritin: acquisition of contrast agents for nuclear magnetic resonance imaging. The apoferritin molecule harbours a trapped manganese oxyhydroxide particle on being subjected to chemical treatment. The subsequent reduction process, above, causes a large number of chemical compounds containing manganese ions to form inside the apoferritin. In this way, high sensitivity contrast agents are obtained for nuclear magnetic resonance imaging in medicine.
Future challenges
In this article, I have tried to illustrate by way of example the huge potential of molecular nanoscience as a discipline, which is giving rise to new chemistry, new physics and new materials. In many ways this field is in its infancy. Thus, today’s chemists and biologists know how to design increasingly complex molecules and how to use molecular recognition and self-assembly processes to create supramolecular associations, or to design new functional molecular materials. However, controlled organization of molecules on surfaces, connection to electrodes and, above all, research into the properties of these individual molecules still represent a challenge; accordingly, how to develop applications in molecular electronics, based on the individual properties of molecules, remains a mystery.
«The potential of molecular nanoscience as a discipline is huge. It is giving rise to new chemistry, new physics and new materials»
With regard to designing new types of molecular materials, chemistry has made significant progress in obtaining materials with electrical, magnetic and optical properties of interest; in fact the first real applications of these materials are beginning to be developed (such as chemical sensors, light emitting devices, biomedical contrast agents…). However, the greater the structural and electronic complexity of the material, the more difficult it is to obtain. In fact, the crystal engineering needed to design a multifunctional material is in its infancy, although it is already beginning to be fruitful both in terms of creating new types of multifunctional materials (conducting magnets, paramagnetic superconductors, chiral magnets…) and in the observation of new physical phenomena. Chemists’ imagination and their passion to create increasingly complex objects will undoubtedly provide many more examples of this type in the future. Obviously, accomplishments in this area rely heavily on the joint efforts of chemists, physicists, biologists and engineers alike. In fact, thanks to the long tradition of collaboration between european groups, Europe finds itself at the forefront of research in the field of multifunctional molecular materials, ahead of Japan and the United States. Spain holds a prominent position in this scenario. In fact, Spanish groups lead many of the initiatives related to developing the magnetic aspects of molecules and materials in Europe.
Bibliography
Clemente-León, M. et al., 2007. «Permanent magnetism in apoferritin-encapsulated Pd nanoparticles». J. Mater. Chem., 17: 49.
Coronado, E. et al., 2000. «Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound». Nature, 408: 447.
Coronado, E. i P. Day, 2004. «Magnetic molecular conductors». Chem. Rev., 104: 5419.
Kálmán, F. K. et al., 2010. «Reduction/Dissolution of a b-MnOOH Nanophase in the Ferritin Cavity to Yield a Highly Sensitive, Biologically Compatible Magnetic Resonance Imaging Agent». Angew. Chem., 49: 612.
Martínez, R. V. et al., 2010. «Large Scale Nanopatterning of Single Proteins used as Carriers of Magnetic Nanoparticles». Adv. Mat., 22(5): 588-591.





