Looking for the origin of life in cosmochemistry
Els asteroides i els seus meteorits rics en carboni

Carbonaceous chondrite meteorites are carbon-containing fragments of primitive asteroids that have offered the only samples available to date giving insights into chemical evolution in laboratory analyses. Their study has revealed that abundant organic chemistry came to be in the Solar System ahead of terrestrial life and, by the input of these meteorites and comets, might have aided in the origin of our planet’s biochemistry.
Keywords: biogenic elements, carbonaceous meteorites, chemical evolution, chirality, chembiogenesis.
Louis Lerman (2010) recently wrote:
Origin problems are the most conjectural and qualitative of scientific questions. It is hardly surprising then that the origin of life, like the origin of the universe, lacks uniquely defining quantitative assumptions and initial conditions. Individually, they are Fermi questions of a functional sort, requiring the use of a first-principles conceptual approach to a situation of fundamental quantitative ignorance.
Well put, if not sobering, since the processes that led to the origin of life remains utterly unknown as well as difficult to decipher.
«That a natural pre-biotic chemical evolution could reach early Earth has found direct experimental scrutiny in the laboratory through the analyses of a group of meteorites containing abundant organic carbon»
Nevertheless, the study of origins have proceeded undaunted, and that intimidating ignorance has been somewhat tempered by sharing it among a wide range of research fields, such as astrophysics, cosmochemistry, and evolutionary biology, just to name a few. This wide perspective has inquired beyond life’s origin and brought to current understanding that biological evolution, through which all organisms derive from a minimal and common ancestor, was itself preceded by chemical evolution. By this process, the biogenic elements H, C, N, O, P, and S originating from the Big Bang and subsequent generation of stars, evolved through a long cosmic history into increasingly complex abiotic molecules, some of which eventually reached planetary systems such as our own and may have led to life’s precursor molecules.
The concept of chembiogenesis
That a natural pre-biotic chemical evolution could reach early Earth, an idea also cleverly referred to as chembiogenesis by Von Kiedrowski (2005), has found direct experimental scrutiny in the laboratory through the analyses of a group of meteorites containing abundant organic carbon, the carbonaceous chondrites. On the basis of these findings and the above-mentioned consortium of studies, in 1985, NASA had already published (Wood & Chang, 1985) the eye-catching summary cartoon shown in Figure 1, which takes us through an evolutionary cosmic path of almost ten billion years (and where, admittedly, the addition of a DNA strand might have been a stretch).
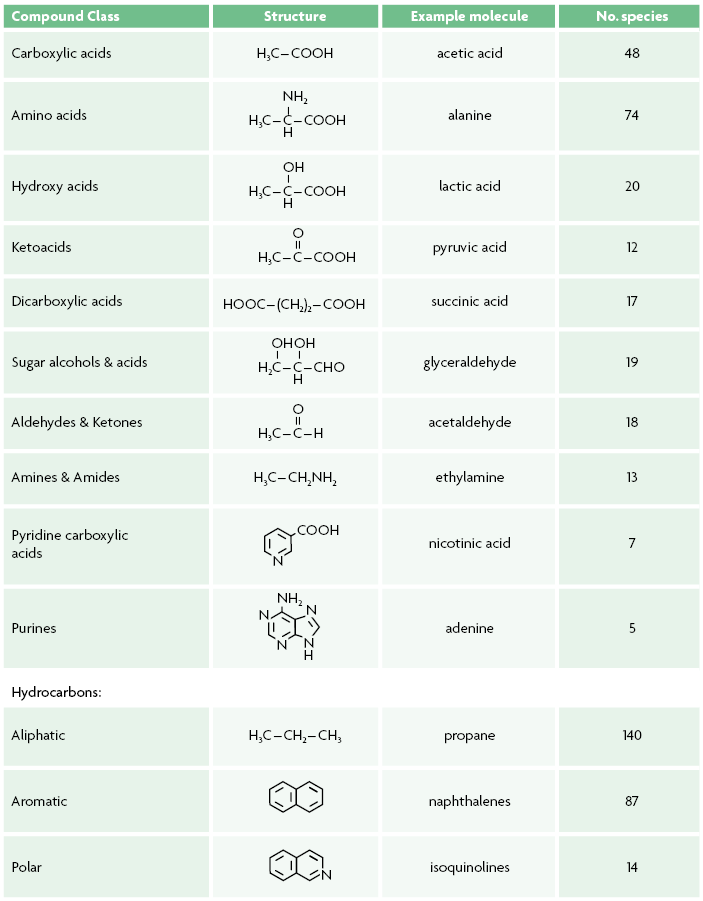
Table 1. Summary of the most important molecule types in carbonaceous chondrites.
Some background may be useful: a meteorite is any small, solid body reaching the Earth from outer space, for example, meteorite samples originating from the Moon, Mars, and even Mercury have all been collected. However, their largest input comes from the Asteroid belt. Asteroids are small rocky bodies that, while falling into an accretion orbit during the early stages of Solar System’s formation, came under the strong gravitational tug of the already formed giant planets, never succeeded in making a planet of their own and remained, in their thousands, orbiting the Sun as aggregates of rocks between Mars and Jupiter. All meteorites reach the Earth following some disruptive impact that destabilizes their original location, but the Asteroid Belt is so crowded that collisions are also very frequent and have put asteroid fragments into Earth’s orbit since the accretion of the planet.
«Studies of these meteorites offer not only proof that compounds identical to life’s molecules formed abiotically, but also insights into the organic inventory that was likely accreted by the early Earth»
Carbonaceous chondrites have a primitive composition and display solar-like elemental distributions depleted only, and to a limited degree, of the most volatile elements H, C, O, and N. These meteorites are rare and comprise only 4.7 % of total falls since 1806; many more are found (which, in fact, are called «finds») but, with the exception of some collected in Antarctica, they usually become quickly contaminated by the chemistry of the biosphere and, since bacteria in particular like them as food, they eventually decompose in terrestrial environments. Carbonaceous chondrites are classified in several subgroups on the basis, inter alia, of water content, hydrous phases within their minerals, and inclusion differences (Zolensky et al., 1997).
All carbonaceous chondrites contain organic carbon, ranging in complexity from macromolecular insoluble organic materials similar to terrestrial kerogens to simple soluble compounds, several of which are identical to biomolecules. Therefore studies of these meteorites offer not only proof that compounds identical to life’s molecules formed abiotically, but also insights into the organic inventory that was likely accreted by the early Earth. Table 1 gives an overview of the major molecular species of compounds found in carbonaceous chondrites and their distribution, but many more are known to be present, if only by their elemental composition (Schmitt-Kopplin et al., 2010).
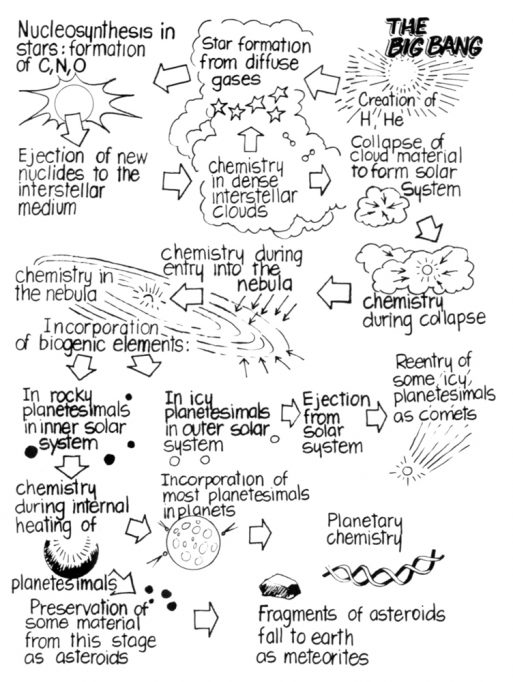
Figure 1. In 1985, NASA published this eye-catching infographic cartoon that takes us through an evolutionary cosmic path of almost ten billion years (and where, admittedly, the addition of a DNA strand might have been a stretch). / Reproduced with permission from Wood and Chang (1985).
The compounds listed in Table 1 (Pizzarello, Cooper, & Flynn, 2006; Pizzarello, Williams, Lehman, Holland, & Yarger, 2011) are usually found after extraction of powders from meteorites with water and solvents; however, free compounds can be also released from the insoluble organic materials, under hydrothermal conditions (300 ˚C and 100 Megapascal) not dissimilar from those found in terrestrial deep ocean vents (Yabuta, Williams, Cody, Alexander, & Pizzarello, 2007). This treatment may produce suites of hydrocarbons as well as oxygen-containing molecules such as dicarboxylic acids and, most surprisingly, large amounts of ammonia (Pizzarrelo & Groy, 2011).
In spite of the extensive molecular and isotopic studies conducted over forty years that have contributed greatly to our knowledge of the distribution, properties, and likely conditions for the formation of meteoritic organic compounds, a comprehensive understanding of the synthetic pathways and even their basic origin environment (i.e., pre-solar vs. solar) is still not fully known for some of them.
Even more elusive is the assessment of a possible exobiology, i.e., how the exogenous input of meteoritic materials might have led to the origins of life. As Figure 1 and Table 1 suggest, the formation of molecules composed of C, H, O, and N appears to be a universal evolutionary process that leads to large chemical complexity, but this occurs in fundamentally distinct ways before and after the onset of life. Prebiotic evolution, in fact, shows traits of a chemistry governed solely by physicochemical forces while extant terrestrial life, be it simple or complex, demonstrates a strict chemical selectivity in the structure and function of its polymeric as well as monomeric constituents. Comparative examples can be found throughout biochemistry, for example, meteoritic amino acids have almost complete structural diversity for a given carbon number, showing decreasing abundances with the lengthening of their carbon chains, and nearly one hundred different ones can be found, while terrestrial proteins only contain 22 components (Pizzarello, Cooper, & Flynn, 2006).
Biochemistry from meteorites?
Another theme in chembiogenesis is the study of whether any of the chemical traits of terrestrial biochemistry could derive from cosmochemistry. In the search for a possible relationship between meteorite organics and biochemistry, molecular asymmetry stands out. Chirality, from the Greek ceir for hand, is a general property of objects that, like the hands, can exist in two forms that are mirror images of each other but are non-superimposable. This is also a property of any organic molecule with a carbon and four different substituents, known as an asymmetric carbon (C* in Figure 2). The two mirror image forms of a chiral compound are referred to as enantiomers and, in the case of biochemical compounds such as amino acids and hydroxy acids, they are referred to as D- and L- enantiomers (from Latin dexter and laevus).
«The data available so far certainly allows us to draw a plausible scenario, where exogenous materials such as those of meteorites and, likely, comets were abundantly delivered to the early Earth and contributed to the planet’s organic pool»
Chirality is first and foremost an interactive property, as can easily be visualized by thinking of two people shaking their right hands rather than their right and left ones. On this basis, it is fundamental accumulation of life’s structures and functions; for example, all amino acids in terrestrial proteins have the L-configuration while the sugar of information polymers, DNA and RNA, is always a D-enantiomer. Chirality, therefore, is an indispensable molecular trait of some essential biomolecules.
Given the stochastic distributions determined for all meteorite organics, it was unexpected and quite exciting when some amino acids in meteorites were found to have an excess of L-enantiomers. At first this research was controversial because of the «homochirality» of terrestrial proteins and the ease of terrestrial contamination, but they were eventually detected for some compounds that are abundant in meteorites but not common in terrestrial biochemistry, the α-methyl-α-amino acids, and excesses of enantiomeric (ee) were observed that, even though they were not as extensive, had the same configuration (L-) as terrestrial amino acids (Pizzarello & Groy, 2011). To date, ten of these compounds and one hydroxy acid have shown to be ee and the findings have raised anew the speculations that extraterrestrial prebiotic processes might have provided the early Earth with a «primordial» inventory of essential organic molecules, giving molecular evolution a push due to their chiral asymmetry, i.e., this has brought about speculation about possible exobiology.
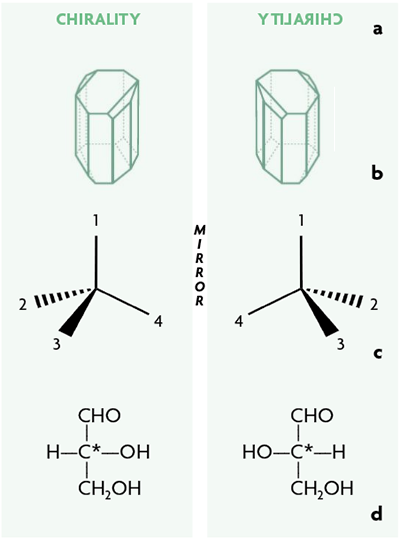
Figure 2. Chirality is a general property of objects that can exist in two forms that are mirror images of each other but are non-superimposable. It may be the property of assemblages of various components (a), crystals (b) and any organic molecule with a carbon and four different substituents, referred to as an asymmetric carbon (c-d).
Among the possible effects of the exogenous delivery of carbonaceous material, the extraction of vesicle forming molecules from the Murchison meteorite may also be of particular significance (Figure 3). In 1985 Deamer demonstrated that boundary structures are formed from organic components extracted from the Murchison meteorite, such as a mixture of amphiphilic components and longer chain carboxylic acids among others, which efficiently collaborate in water solutions to give a sustained increase in the surface tension toward the formation of membranes and vesicles (Deamer, 1985). These findings raise the interesting prospect that meteoritic components could have contributed to the self-assembly capabilities of the early Earth milieu.
To conclude this short overview of the chemistry of carbonaceous meteorites, we could ask whether the title of the article is reasonable and we can, indeed, come to think that cosmochemistry contributed to life’s origin. If constrained by the many unknowns that surround this origin, the data available so far certainly allows us to at least draw a plausible scenario, where exogenous materials such as those of meteorites and, likely, comets were abundantly delivered to the early Earth, contributed to the planet’s organic pool, provided incipient means of selection through some of their chiral compounds and catalysts which contributed to prebiotic molecular evolution.
Given the large and overwhelming unknowns of this quest, it has been somewhat reassuring to find that, by 6 January 2015, the Kepler mission had confirmed the 1,000th exoplanet candidate and that six of the newly confirmed exoplanets are near-Earth-sized and found in their host star’s habitable zones… Galileo may not have been thinking of exobiology in stating that astra regunt, but despite this, looking at the stars may still provide us with basic information after all.
References
Deamer, D. (1985). Boundary structures are formed by organic components of the Murchison carbonaceous chondrite. Nature, 317, 792–794. doi: 10.1038/317792a0
Lerman, L. (2010). The primordial bubble: Water, symmetry breaking, and the origin of life. In R. M. Lyndell-Bell, S. C. Morris, D. Barrow, J. L. Finney, & C. Harper. (Eds.), Water and life: The unique properties of H2O (pp. 259–290). Boca Raton: CRC Press.
Pizzarello, S., Cooper, G. W., & Flynn, G. J. (2006). The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In D. Lauretta, & H. Y. McSween Jr (Eds.), Meteorites and the Early Solar System II (pp. 625–651). Tucson: University of Arizona Press.
Pizzarello, S., & Groy, T. L. (2011). Molecular asymmetry in extraterrestrial organic chemistry: An analytical perspective. Geochimica et Cosmochimica Acta, 75, 645–656. doi: 10.1016/j.gca.2010.10.025
Pizzarello, S., Williams, L. B., Lehman, J., Holland, G. P., & Yarger, J. L. (2011). Abundant ammonia in primitive asteroids and the case for a possible exobiology. Proceedings of the National Academy of Sciences, 108, 4303–4306. doi: 10.1073/pnas.1014961108
Schmitt-Kopplin, P., Gabelica, Z., Gougeon, R. D., Fekete, A., Kanawati, B., Harir, M., … Hertkorn, N. (2010). High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proceedings of the National Academy of Sciences, 107, 2763–2768. doi: 10.1073/pnas.0912157107
Von Kiedrowski, G. (Org.). (2005, September 28–October 1). Chembiogenesis: Prebiotic chemistry and early evolution. ECLT: Venice International University. Retrieved from http://www.istpace.org/Web_Final_Report/scientific_meetings_at_eclt/workshops/second_year_april_2005_marc/prebiotic_chemistry_and_ear.html
Wood, J. A., & Chang, S. (Eds.). (1985). The cosmic history of the biogenic elements and compounds. Washington, DC: Scientific and Technical Information Branch, National Aeronautics and Space Administration Publisher.
Yabuta, H., Williams, L. B., Cody, G. D., Alexander, C. M. O’D., & Pizzarello, S. (2007). The insoluble carbonaceous material of CM chondrites: A possible source of discrete organic compounds under hydrothermal conditions. Meteoritics & Planetary Science, 42, 37–48. doi: 10.1111/j.1945-5100.2007.tb00216.x
Zolensky, M. E., Mittlefehldt, D. W., Lipschutz, M. E., Wang, M.-S., Clayton, R. N., Mayeda, T. K., … Barber, D. (1997). CM chondrites exhibit the complete petrologic range from type 2 to 1. Geochimica et Cosmochimica Acta, 61, 5099–5115. doi: 10.1016/S0016-7037(97)00357-8





