Surviving uncertainty
Biodiversity, adaptation, and environmental fluctuation in rotifers

Studying evolution in the face of environmental uncertainty is crucial to understand biological diversity, because diversifying life strategies is key to survival and reproduction in uncertain environments. Rotifers are planktonic microinvertebrates that live in inland water bodies. Their complex life cycle combines sexual and asexual reproduction and, together with their small size and short generation times, makes them excellent model organisms in evolutionary ecology studies. Here we present field population and laboratory experimental evolution studies which show that these organisms can adapt by locally diversifying their life cycle to face unpredictable fluctuations in their environment.
Keywords: plankton, rotifers, complex life cycles, temporal environments, environmental unpredictability.
«Adaptation implies specialising and improving skills related to certain conditions»
Introduction
If an organism were able to adapt to any biospheric circumstance – i.e., if it could survive in the tropics and the poles, in water and the atmosphere, taking advantage of any biotic or abiotic energy source – it would probably be the only living being on Earth and the diversity of organisms in our planet would be minimal. In practice, however, adaptation implies specialising and improving skills related to certain conditions, sacrificing some abilities for others. Therefore, adaptation is just one «dimension» in explaining biodiversity, rather than the only one. Adaptation is required for a species’ survival in a particular location and, consequently, in a habitat or region, and in the biosphere. Darwinism teaches us that adaptation occurs thanks to natural selection. Just as any other natural force, it acts as what an Aristotelian would call an «efficient cause»,1 coming from the past with no particular purpose. That is to say, when a living being finds itself in an environment that is somewhat «genuinely» new, we cannot expect it to already be adapted to it. At best, it will enter a process of natural selection that will end with it adapting to the novel environment. Because all environments change, this represents a crucial challenge for the adjustment of organisms to their environments.
«The life cycle of many rotifers involves cyclical parthenogenesis, consisting in the combination of sexual and asexual reproduction»
To ponder upon the ubiquity of environmental changes, we just need to think about climate variations and notice all the different sorts of clothes we keep in our wardrobe (summer clothes, winter clothes, etc.) or remember how we decide what to take on an excursion. These two examples illustrate cases of «environmental fluctuations» in which the conditions recur frequently, contrasting with «environmental novelties», such as climate cooling on a geological scale or the appearance of a new pathogen. Continuing with the simile: we do not store clothes in anticipation of a glacial age. In the case of fluctuations, the recurrence occurs within the time scale of organisms’ evolutionary response. Thus, it can act as a selective pressure factor, with the adaptation evolving to meet not only the average environmental conditions, but also their potential variability.
«While adapting to environmental uncertainty is important under any circumstances, in the future it will probably be essential»
In this paper, we address a particular type of environmental fluctuation. The examples of the wardrobe and the excursion illustrate two very different types. Because of their periodicity or prior signals, some fluctuations can be predicted by the organisms. Others cannot. However, thanks to natural selection, the mean and variance of an unpredictably fluctuating factor (the latter being the most common statistics to quantify variation) can be enough to optimise the characteristics of an organism (when understanding optimisation as a maximisation of biological performance, which is often referred to as fitness). Evolutionary theory states as much, and our empirical studies, as we will see below, confirm it. But before delving into these, let us first warn the readers about a second-order complication: one of the environmental novelties that can be expected as a result of global change is an increase in fluctuations, as well as in their unpredictability. While adapting to environmental uncertainty is important under any circumstances, it will probably be essential in the future.
Risk minimisation strategies
For an organism that needs to complete its life cycle in a year, leaving many descendants most years while being unable to leave any for a few other years would be worthless. Those annus horribilis would represent an impassable obstacle for the species. Considering an entire series of years, its biological fitness would be zero. Therefore, the arithmetic mean of fitness in the different environmental situations (which is often used to describe individual reproductive success), is not an appropriate measure in this case. In unpredictable environments, we expect to find adaptations that will not help individuals maximise their average fitness; evolutionary predictions cannot be made without considering the variance in it. Thus, when unpredictable changes must be anticipated, we expect organisms to evolve risk-minimising strategies (bet hedging) that will reduce the variance in fitness at the cost of also reducing its arithmetic mean, but increasing its geometric mean; that is to say, they maximise long-term biological fitness.
«Due to the time scale at which it operates, the adaptive response of living beings is not easy to study»
Evolutionary theory has approached this problem in several ways. A simple one that still captures the essence of the problem assumes that an organism can face two types of conditions (favourable or adverse), and that these follow each other in an unpredictable series. One way to minimise risks is to possess constant traits that work well independently from environmental conditions. These traits would allow the species to survive adverse conditions at the cost of not fully exploiting the favourable ones, following a conservative strategy. Another way to minimise risks is to produce a split between descendants adapted to adverse conditions and those adapted to favourable conditions. The objective would be to increase the probability of success with a diversifying strategy. Thus, Cohen (1966) analysed the situation of an annual plant that spends the winter in seed form and germinates as spring approaches, with no information about whether the year will allow it to reach the flowering and fruiting stages. His prediction was that some of the seeds produced by the adult plants would germinate (in case the year is good), while the rest would remain dormant (in case the year is bad), and that this divided structure would repeat year after year for the seeds that survived in dormancy in the previous year. The existence of dormant seed banks in the soil supports this prediction.
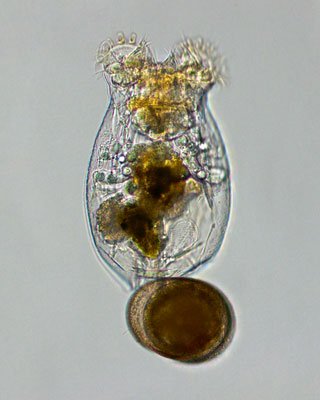
Figure 1. Microphotograph of a rotifer female with a diapausing egg. These tiny invertebrates are usually part of the plankton present in lakes and lagoons and their complex life cycle makes them excellent model organisms for evolutionary ecology studies. / Ivana Jezkova
Rotifers, model organisms in ecology and evolution
Because of the time scale on which they operate, an organism’s adaptive response is not easy to study. We must either resort to indirect inference or to special experimental systems. One such system is that of rotifers (Serra, García-Roger, Ortells, & Carmona, 2019), a phylum of small invertebrates (0.1–0.4 mm in length; Figure 1) that are often part of the plankton of lakes and lagoons. Their most interesting feature is their life cycle: in many cases, cyclical parthenogenesis. Cyclical parthenogenesis (Table 1) is the combination of sexual and asexual reproduction. The latter, also known as parthenogenesis – literally, reproduction by virgins2 – is based on the reproduction of females who, except in the case of mutations, are identical genetic copies of their mothers. With no need to invest in males for mating, and combined with the small body size of these organisms, asexual reproduction results in high proliferation rates (a population can double in size in a couple of days), which allows them to rapidly colonise habitats with large populations. This is critical because the rotifer habitat is viable only for a part of the annual cycle, the so-called growing season, after which the active population disappears. Before the end of the growing season, there must be at least one sexual generation which, in many species, is induced by overcrowding to a certain population density. Males are then born that copulate with the females and the resulting eggs enter diapause and remain in the lagoon sediment in conditions that would not be survivable for active individuals. The dormant phase, which can be quite prolonged, then begins. Therefore, the timing of sexual reproduction among rotifers is critical. It must happen late enough that it does not compromise asexual proliferation and early enough for them to provide diapausing eggs in time. But of course, no two years are identical in terms of the growing season duration: this length of time is uncertain.
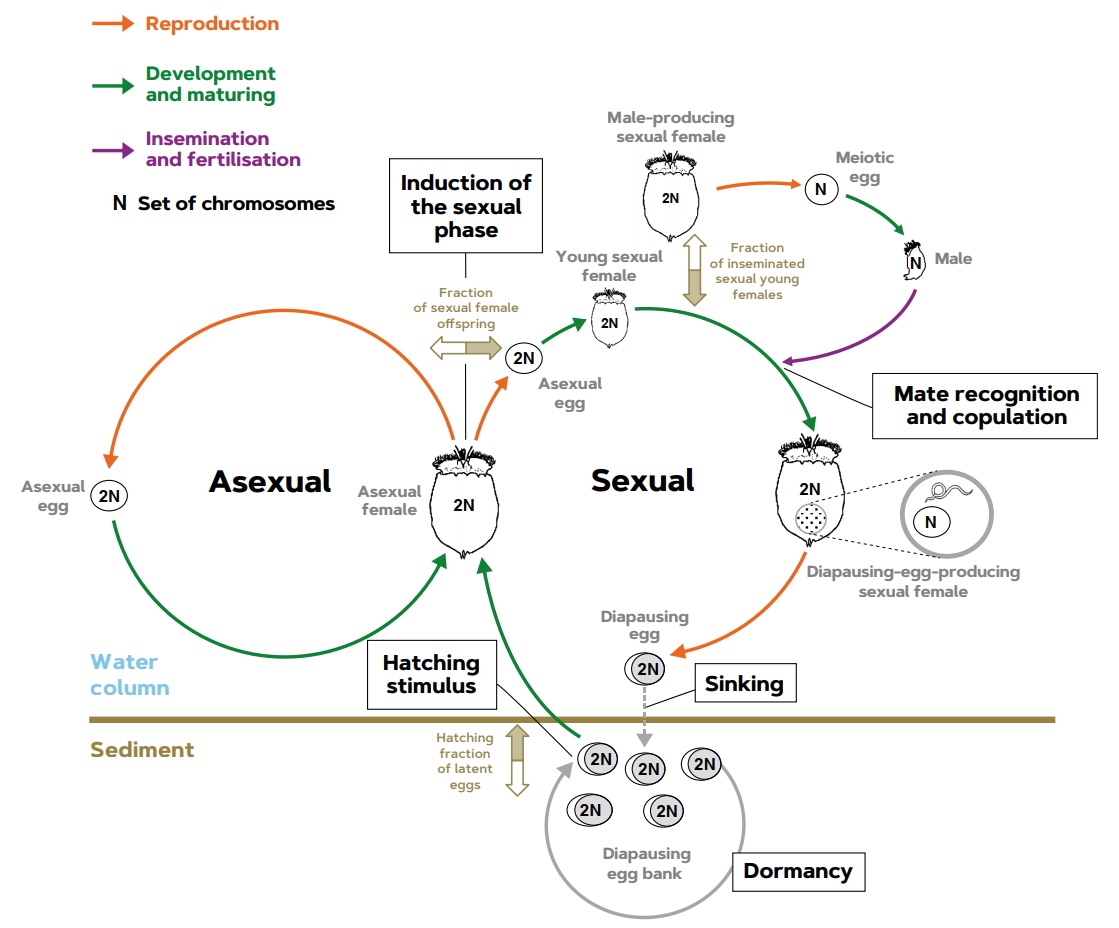
Diagram 1. Cyclical parthenogenetic rotifer populations are usually temporary in plankton and they recolonise the water column during the planktonic growing season by hatching diapausing eggs from the sediments of lakes and lagoons. Recently hatched individuals are asexual females that produce diploid eggs (with two chromosome sets) that – barring mutations – develop into daughters that are genetically identical to their mothers. This exclusively asexual reproduction is repeated an indeterminate number of times (clonal proliferation). The sexual phase – which does not completely stop asexual reproduction – starts with the parthenogenesis of sexual daughters from asexual mothers as a fraction of their offspring, in response to inducing environmental factors. Sexual females produce eggs with a single chromosome set (haploids) resulting from meiosis (cell division in which the gene endowment is halved), which develop into haploid males or, if young sexual females mate, their haploid eggs are fertilised and become embryos, which enter into diapause (a kind of dormancy). These eggs are deposited in the sediment, can survive adverse conditions, and allow the populations to recolonise the water column when adequate conditions return, and also allows them to spread to other habitats. After receiving appropriate stimuli, some of the diapausing eggs hatch into asexual females thus, starting a new growing season. The diapausing eggs that do not hatch accumulate, forming egg banks in the sediment. / Source: Modified from Serra et al. (2019).
Cyclical parthenogenesis represents a delicate adjustment between the advantages and disadvantages of sexual reproduction and its associated trait in these animals: dormancy. This life cycle deserves study and has been used to research evolutionary hypotheses about the advantages of sexual reproduction and the divergence between local populations, as well as ecological hypotheses about the coexistence of species (Serra et al., 2019). In addition, genetic and environmental factors can easily be distinguished in the life cycle of rotifers because asexual reproduction generates genetically identical clones. Finally, the high rate of asexual proliferation involves short generation times. This, together with rotifers’ small size, allows for experimental evolution studies; i.e., studies to check whether laboratory populations, created by researchers under controlled conditions, respond to selection pressures in similar ways to populations in their natural habitat.
Studies with Iberian rotifer populations

Figure 2. In the picture, sampling in the Hoya Rasa lagoon, included in the ICBiBE Evolutionary Ecology Laboratory studies. Active rotifer populations are sampled in the water column with a zooplankton net (in the picture background), and their dormant forms (diapausing eggs) are sampled in the sediment using a Van Veen grab. In the foreground of the picture, a researcher collects the sediment extracted using the grab. / María José Carmona
The climate of the Mediterranean region is characterised by irregular variations in the precipitation and evaporation cycle. Unsurprisingly, this variation affects the hydrological characteristics of small bodies of water such as the lagoons in the region of La Mancha (Figure 2) in the east of the Iberian Peninsula. To study this region, Lluís Franch-Gras and colleagues (Franch-Gras, García-Roger, Franch, Carmona, & Serra, 2017) from the Evolutionary Ecology group of the Cavanilles Institute of Biodiversity and Evolutionary Biology at the University of Valencia (ICBiBE) adapted a set of methods to analyse satellite images. The goal was to detect water bodies of variable sizes. This allowed them to take advantage of data about an unusually long time series in ecology. The authors used this analysis to characterise the fluctuations of the annual flood periods of the lagoons (their hydroperiod), revealing that these periods are very variable: some lagoons are very predictable and others very unpredictable.
«If an organism were able to adapt to any biosphere circumstance, it would probably be the only living being on Earth»
Based on these observations, an initial adaptive hypothesis was established (Franch-Gras, García-Roger, Serra, & Carmona, 2017) in which populations of rotifers living in very unpredictable lagoons had an early onset of sexual reproduction in order to produce diapausing eggs early as well as to successfully survive an unexpectedly short growing season. However, this consequently decreases asexual reproduction which would otherwise allow them to more fully exploit potentially long growing seasons. In a second hypothesis, very similar to theories related to annual plants, these researchers proposed that rotifers in lagoons with an unpredictable hydroperiod might possess genes that would allow them to produce a high proportion of diapausing eggs that would not hatch as soon as the environmental conditions seemed favourable. Thus, diapausing eggs would be banked in the sediment in case the growing season was too short to complete the reproductive cycle with a new cohort of diapausing eggs. This would be at the expense of losing competitive capabilities if the growing season did allow the cycle to be completed. These predictions imply that there is genetic differentiation between populations of the same species, which would involve local adaptation. Testing of these hypotheses, therefore, was best done by keeping the rotifers from the different lagoons under laboratory conditions, controlling the non-selective environmental effects that affect their development.
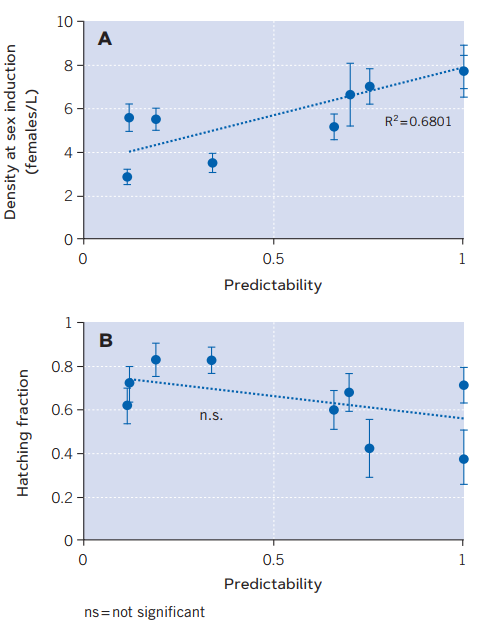
Figure 3. Relationship between two diapause-related traits in nine natural populations of the cyclical parthenogenetic rotifer Brachionus plicatilis and the degree of unpredictability of the lagoons they inhabit. Figure A, onset of sexual reproduction, inferred from the population density values which induce it. When sexual reproduction is induced at lower densities, the earlier onset of this type of reproduction which guarantees the production of diapausing eggs by these rotifers is indicated. Figure B shows the hatching fraction of diapausing eggs (in parts per unit). A lower fraction guarantees survival if the environment is unfavourable. For each trait, the figure shows the average values in each population and its error bars (± 1 standard error). The dashed line in each graph represents the minimum-quadratic regression adjustment. The coefficient of determination (R2 = 0.6801) is the proportion of the variance in the density at sexual reproduction induction explained by environmental predictability. / Source: Modified from Franch-Gras et al. (2019).
The results showed that the genotypes from environmentally unpredictable lagoons did initiate sexual reproduction earlier than those from more predictable environments (Figure 3a). However, no evidence was obtained in favour of the second hypothesis (Figure 3b), perhaps because one trait – the starting point of sexual reproduction – is sufficient to achieve the necessary risk minimising strategy, or perhaps because the heterogeneity of conditions in the sediment is so high that an intrinsic trait – such as the hatching fraction – is ineffective (Franch-Gras, García-Roger, Serra, et al., 2017).
The evidence provided by the studies carried out in our laboratory (Franch-Gras, García-Roger, Franch et al., 2017; Franch-Gras, García-Roger, Serra et al., 2017) is observational, which implies that relationships are described in their natural context – they are realistic. However, it is still worth asking if those relationships might not be causal; i.e., if the characteristics of the life cycle differ between populations as the result of some other selective pressure associated with environmental unpredictability. To rule this out, experimental evolution studies were carried out (Tarazona, García-Roger, & Carmona, 2017). In our laboratory, we created environments with random hydroperiod length fluctuations, as well as environments with constant hydroperiod lengths, and rotifer populations grew in them according to a series of flood cycles. Rotifer biology allowed to simulate these cycles using a chemostat device in a room with controlled conditions. The results corroborated our predictions and also showed that the expected adaptive divergence occurred rapidly. Laboratory populations evolved toward the earlier onsetof sexual reproduction under unpredictable conditions (Figure 4a). This observation was consistent with the conservative risk-minimising strategy suggested by the results of the natural populations study (Figure 3a). In addition, compared to the predictable regime, diapausing egg hatching in the unpredictable regime populations was also lower (Figure 4b). Other studies have recognised the rapid evolution of rotifers (Declerck & Papakostas, 2016). In this case, sexual reproduction combined with clonal selection operating on an individual’s entire set of genes were important factors. Our system is undoubtedly influenced by the fact that the initial experimental population comprised several clones from various natural populations. Unlike in our field observations, both our predictions were fulfilled in the experimental evolution studies. This is probably because the laboratory conditions in which diapausing eggs hatch are much more homogeneous than those in nature where genotypes express themselves more accurately and the hatching proportion of rotifers are a good target for selective evolution.
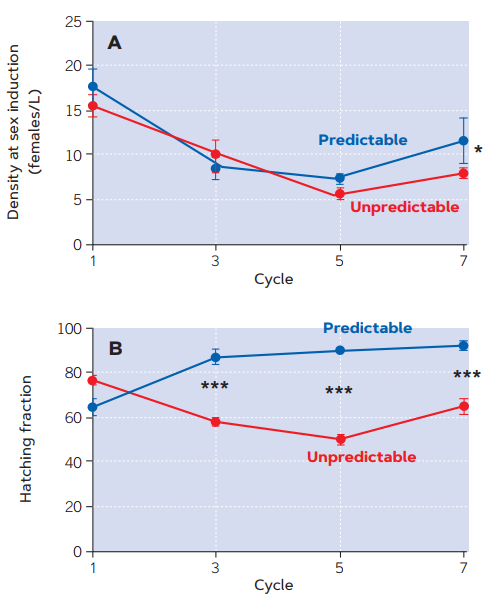
Figure 4. Evolution of two diapause-related traits in laboratory populations of Brachionus plicatilis over seven selection cycles in divergent environmental (hydroperiod duration) fluctuation regimes: predictable (constant hydroperiod duration over each growing cycle) and unpredictable (variable hydroperiod durations over the growing cycles). Figure A shows the onset of sexual reproduction, inferred from population density values throughout the experimental evolution cycles. A lower density value for the induction of sexual reproduction indicates an earlier start. Thus, in more unpredictable conditions, the onset of sexual reproduction usually comes earlier. Figure B shows the hatching fraction of diapausing eggs (percentage) over the cycles of experimental evolution. The hatching fraction is lower in the unpredictable cycles (i.e., a higher fraction of eggs remain dormant in diapause to keep them safe from potentially unfavourable conditions). For each trait, the average values for each regime and its error bars (± 1 standard error) are shown. Asterisks indicate statistically-significant differences between the regimes (* p < 0.05; ** p < 0.01; *** p < 0.001). / Source: Modified from Tarazona et al. (2017).
The design of these aforementioned studies (a combination of field and laboratory work) allows to unambiguously know that the differences in life cycle traits are genetic, i.e., that the differences are not distorted by variations in the environment in which the genes are expressed. These results provide strong evidence that these differences in life cycle are caused by natural selection in response to environmental unpredictability; that is, because of adaptive evolutionary divergence. Even so, these studies did not succeed in identifying the specific genes responsible. However, the recent development of genomic analysis technologies – both by sequencing genetic material and by bioinformatics analysis – allows progress in this latter sense, even for species that were not the subject of intensive genetic study.
Thus, a «draft» rotifer genome was created from sequencing data in a new study (Franch-Gras et al., 2018) and compared to previously sequenced populations that were differentially adapted to environmental unpredictability in order to analyse divergence between these genomes. Using genotyping by sequencing (GBS) and subsequent bioinformatic analysis, genetic divergence (single nucleotide polymorphisms, SNPs) were found in a large number of nucleotide positions, even when applying very conservative methods. Tarazona and colleagues also followed this procedure with the laboratory populations that had diverged in their experimental evolution trials. In both studies, several SNPs – most of them located within genes – were present in a higher proportion than expected at random and correlated with environmental unpredictability and studied life cycle traits. Therefore, these genes may be responsible for local adaptation to unpredictable environments. While the functional relationship between these genes and life cycle traits is unclear, they represent a starting point for studies that belong more to the fields of molecular and cellular biology than to evolutionary ecology.
Overall, the results show that rotifer populations can develop adaptive responses in diapause-related traits to adjust to the local degree of environmental unpredictability. These responses involve different sorts of risk-minimisation strategies. Studies conducted in the Evolutionary Ecology Laboratory at ICBiBE provided an analysis of these populations’ evolutionary responses through an integrated approach that involves mapping the genotype (genetic markers) to the phenotype (life cycle traits), as well as the phenotype in the environment (habitat characteristics).
«Some evolutionary forces can oppose natural selection and make adaptation difficult»
Preferential mating and local adaptation protection
Some evolutionary forces can oppose natural selection and make adaptation difficult. For the aforementioned rotifer populations, which inhabit lagoons that are very close to each other, we need to consider the effect that the arrival of individuals from lagoons with different predictability characteristics would have on the resident population. These individuals would not be adapted to their new environment, but they might still have an opportunity to meet local residents at the time of sexual reproduction. This exogamy would blur adaptive divergence and involve a loss of effectiveness for the resident population. This scenario led to the formulation of the «reinforcement hypothesis», which is very important in speciation theory (Butlin, 1987). In short, the described process represents a certain reproductive isolation because the exogamous offspring is not adapted to its local habitat. This would cause selective pressure against the residents and interbreeding immigrants, reinforcing the pre-existing isolation. If the isolation deepens sufficiently, divergent populations would become different species, thus generating biological diversity. In the Evolutionary Ecology Laboratory at ICBiBE, Ivana Jezkova’s PhD work aims to verify whether there is any indication of preferential intra-population mating due to environmental unpredictability in divergent rotifer populations. Her preliminary results point in this direction and, if confirmed, would provide evidence for an adaptive divergence stabilisation mechanism against the adaptation «disruption» represented by immigration. These sorts of results are extremely interesting because the reinforcement hypothesis is not trivial. Countering this idea is the fact that sexual reproduction requires coordination of male and female individuals in space and time as well as their identification of each other. This coordination penalises variability.

Figure 5. Chemoreceptors intervene in rotifers’ mate recognition. These are surface proteins that function as signals so the male (smaller than the female) can recognise the females from its own species. The picture shows that the mating process includes (left to right) an encounter, circling the female, and copulation. / Ivana Jezkova
«Genotypes from environmentally unpredictable lagoons did initiate sexual reproduction earlier than those from more predictable environments»
Conclusions
Adaptation is one of the most striking aspects of evolution by natural selection, even though it leaves a trace in the genetic material of living organisms. It can only be completely understood by observing the morphological, physiological, and ethological traits of an organism, and especially, by the characteristics of its life cycle, because these are directly related to biological efficacy (i.e., the characteristics we call «fitness components»). Free from all finalism, this theory explains adaptation in two ways. On the one hand, consistency between the past environment (which selected for certain traits) and the present environment (where the adaptive value of those traits manifests). On the other hand, is the consistency in the biological traits of lineages because of inheritance. This turns adaptation to environmental fluctuation into a more challenging phenomenon – and a more fascinating one to study – especially if such fluctuation is irregular. Thanks to its ability to optimise processes, theory suggests that such adaptation is, in principle, possible. The evolution experiments carried out in the Evolutionary Ecology Laboratory show that it can occur in living beings – such as rotifers – which are committed to many other adaptive needs. In turn, the patterns described by studies with natural populations indicate that the causes identified in the experiments also act on the intertwined factors present in nature. The population-based stabilisation mechanisms and – on a different level – their underlying molecular and cellular mechanisms remain to be clarified. Regarding this latter point, the age of «-omics» sciences (genomics, proteomics, transcriptomics, metabolomics, etc.) is providing a tremendous opportunity to bridge the gap between molecular biology and evolutionary ecology.
«Sexual reproduction requires the coordination of male and female, their spatial and temporal coincidence, and their identification of each other»
The final message is that, in the face of environmental uncertainty, evolution can produce diversity at different levels: intraspecific diversity, favouring the diversification of life strategies (functional diversity) and interspecific diversity, favouring speciation processes (i.e., classical species diversity).
1. In his Metaphysics, Aristotle described four types of answer to our questions about the reasons for things. The material cause is related to the material with which things are made (i.e., wood is the material cause of a table); the formal cause is related to its design (i.e., its shape, layout, or appearance; for instance, a table is formally a flat structure supported by legs); the efficient cause would be related to the interaction with other external agents (in the table example, the carpenter is the efficient cause); and the final cause is related to the purpose and refers to a designer (i.e., the formal cause was the carpenter’s intention to build a table that would be used to eat or study). (Go back)
2. From Greek parthenos (“virgin”) and genesis (“creation”). (Go back)
Some of the work presented here was financed with public funds from the Spanish Ministry of Economy and Competitiveness (projects CGL2012-30779 and CGL2015-65422-P, both co-financed with FEDER funds).
References
Butlin, R. (1987). Speciation by reinforcement. Trends in Ecology & Evolution, 2(1), 8–13. doi: 10.1016/0169-5347(87)90193-5
Cohen, D. (1966). Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology, 12(1), 119–129. doi: 10.1016/0022-5193(66)90188-3
Declerck, S., & Papakostas, S. (2016). Monogonont rotifers as model systems for the study of microevolutionary adaptation and its eco-evolutionary implications. Hydrobiologia, 796(1), 131–144. doi: 10.1007/s10750-016-2782-y
Franch-Gras, L., García-Roger, E. M., Franch, B., Carmona, M. J., & Serra, M. (2017). Quantifying unpredictability: A multiple model approach for Mediterranean ponds by using satellite imagery. PLOS ONE, 12(11), e0187958. doi: 10.1371/journal.pone.0187958
Franch-Gras, L., García-Roger, E. M., Serra, M., & Carmona, M. J. (2017). Adaptation in response to environmental unpredictability. Proceedings of the Royal Society B: Biological Sciences, 284(1868), 20170427. doi: 10.1098/rspb.2017.0427
Franch-Gras, L., Hahn, C., García-Roger, E. M., Carmona, M. J., Serra, M., & Gómez, A. (2018). Genomic signatures of local adaptation to the degree of environmental predictability in rotifers. Scientific Reports, 8(1), 16051. doi: 10.1038/s41598-018-34188-y
Franch-Gras, L., Tarazona, E., García-Roger, E. M., Carmona, M. J., Gómez. A., & Serra, M. (2019). Rotifer adaptation to the unpredictability of the growing season. Hydrobiologia. doi: 10.1007/s10750-019-3886-y
Serra, M., García-Roger, E. M., Ortells, R., & Carmona, M. J. (2019). Cyclically parthenogenetic rotifers and the theories of population and evolutionary ecology. Limnetica, 38(1), 67–93. doi: 10.23818/limn.38.13
Tarazona, E., García-Roger, E. M., & Carmona M. J. (2017). Experimental evolution of bet hedging in rotifer diapause traits as a response to environmental unpredictability. Oikos, 126, 1162–1172. doi: 10.1111/oik.04186
All four authors are members of the ICBiBE Evolutionary Ecology Laboratory whose research focuses on evolutionary ecology and population ecology studies with aquatic microorganisms, more specifically on the demographic, genetic, and ecological analysis of zooplankton. Some of their scientific interests include the adaptation of rotifer life cycles to variable environments, processes mediating the coexistence of competing species, population differentiation and speciation, diapause as a dispersal strategy in time and space, and the evolutionary processes that maintain sexual reproduction in populations.





