Conservation genetics
Applying evolutionary concepts to the conservation of biological diversity
Greater understanding of the forces driving evolutionary change and influencing populations, together with the latest genetic analysis techniques, have helped conserve of biodiversity for the last twenty years. This new application of genetics is called conservation genetics.
Keywords: genetic drift, inbreeding, extinction vortex, effective population size.
One of the most pressing problems caused by human population growth and the irresponsible use of natural resources is the conservation of biodiversity. Species are disappearing at a breakneck pace and a growing number of them require human intervention to optimize their management and ensure their survival. There are various reasons why conservation programmes are necessary, ranging from the purely ecological to the historical, cultural, social and even economic. For example, some local breeds of domestic animals are associated with characteristic production systems or they are the source of a quality product. It is also of great social interest to maintain certain species or breeds that have historically been linked to a region or ethnicity, and form part of their cultural heritage. In the last twenty years, biodiversity loss and species extinction due to human activity have triggered an interest in seeking solutions, both in terms of developing analytical techniques to identify the underlying causes of the problem, and active management techniques to provide solutions. Conservation genetics essentially rests on the foundations of population genetics and classical evolutionary genetics, disciplines studied since the early twentieth century. The study of the genetic makeup of populations and forces of evolutionary change acting on them (i.e., natural selection, genetic drift, mutation and migration; Benito and Espino, 2012) are the pillars supporting conservation genetics. The launch in 2000 of the journal Conservation Genetics, dealing specifically with this field, and more recently, in 2009, of the journal Conservation Genetics Resources highlight the importance of this new application of population and evolutionary genetics.
Genetic variability: gene and allelic diversity
The potential of a population to evolve and adapt to environmental changes depends mainly on genetic variability, ultimately, the gene pool which the population carries. This variability is usually estimated by the frequency of heterozygote members (heterozygosity or gene diversity, Figure 1), i.e., the frequency of individuals bearing different alleles of a given gene, and allelic diversity, in other words, the number of different alleles that are present in the population for a given gene. Gene and allelic diversity are currently estimated using easily identifiable regions of DNA which, generally speaking, are known as «genetic markers». Since variation is the basis of the evolutionary potential of a species, genetic diversity, in its broadest sense, is key to the ability of a species to respond to environmental challenges and thus ensure species survival and perdurance. Therefore genetic diversity forms an essential pillar of genetic conservation.
Mutation, genetic drift and inbreeding depression
The process of mutation (meaning any change in DNA that occurs during the reproductive process) generates new alleles (and new heterozygous individuals), albeit very slowly, which come to form part of the population’s gene pool. However, in reduced population numbers, such as populations of endangered species, the random change in allele frequencies (so-called «genetic drift») is responsible for the loss of many of these alleles with the time-course of generations. The loss of alleles by genetic drift is one of the most pressing issues facing endangered species. Another, no less great, is inbreeding, i.e., the result of mating between relatives, which inevitably occurs when population numbers dwindle. One of the most visible consequences of inbreeding, widely recognized by breeders of animals and plants, is the deterioration of the reproductive capacity or vigour of individuals, a phenomenon called «inbreeding depression». When inbreeding levels rise, many traits – especially those related to reproduction and survival (number of offspring, number of fruits and seeds, viability, fertility…) – are reduced to a greater or lesser extent, depending on the trait and population considered. Inbreeding depression is caused by a combination of two circumstances. First, the majority of mutations in functional genes are deleterious, which means that new alleles generated are generally less functional than the more frequent wild-type allele in the population. Second, these deleterious alleles tend to be recessive, in other words, they only manifest their negative effects on homozygous individuals. Deleterious recessive alleles are usually present in heterozygous individuals that are carriers of the functional wild-type allele, and inbreeding increases the likelihood that these alleles will be present in homozygotes, thus expressing the deleterious effect.
The cheetah provides a classic case of the effects of genetic drift and inbreeding on genetic variation. Some 20,000 years ago, the cheetah’s habitat range covered Africa, Europe and North America, but today its population has declined to between 1,500 and 5,000 individuals, basically in Africa. The estimated genetic diversity of this species is extremely low, and this can give rise to several bottlenecks (drastic reductions in population size) that decimate populations, reducing genetic variability by up to 99% (Menotti-Raymond and O’Brien, 1995). Inbreeding depression in the cheetah is manifested by low sperm counts and the high frequency of abnormal sperm, low fecundity in captivity, high incidence of juvenile mortality (around 30%) and high vulnerability to infectious disease, especially feline peritonitis.
Effective population size
As indicated above there can be various reasons for genetic drift, involving the loss of alleles, and for inbreeding, which involves an increased frequency of homozygotes and thereby inbreeding depression. The most important reasons – though by no means exclusive – are temporary fluctuations in the number of breeding individuals, a bias in sex ratio, or variations in an individual’s contribution to descendants. These circumstances can be quantified by the concept of «effective population size» (Ne), which is defined as the hypothetical or idealised population size if it were free of the cited causes for population drift, which would lead to the inbreeding or drift observed in a real population (Benito and Espino, 2012, chap. 24).
Effective population size of natural populations can be estimated using several methods, but numerous studies suggest that effective population size is usually much lower than the census of reproductive population size (N), i.e., total number of breeding individuals, with the former representing around just 10% of the latter, on average (Frankham et al., 2010). Conservation programmes often aim to increase or maintain a high effective population size, thus the basic question that arises is: What minimum effective population size should a population have to be reasonably protected against genetic drift, and the minimum below which population viability is compromised? Several studies have been conducted in this area and concluded that the minimum effective population required to ensure population sustainability should be around N = 500 individuals. Thus considering the empirical relationship between N and Ne described above, the number of breeding individuals should be 10 times greater, namely N = 5,000. This relatively high number highlights the difficult situation of many plant and animal populations in the wild.

Figure 1. Computer simulation of the evolution of heterozygosity (H: mean frequency of heterozygotes) over generations of five genes with initial frequency of 0.5 in a population with ten breeding individuals.
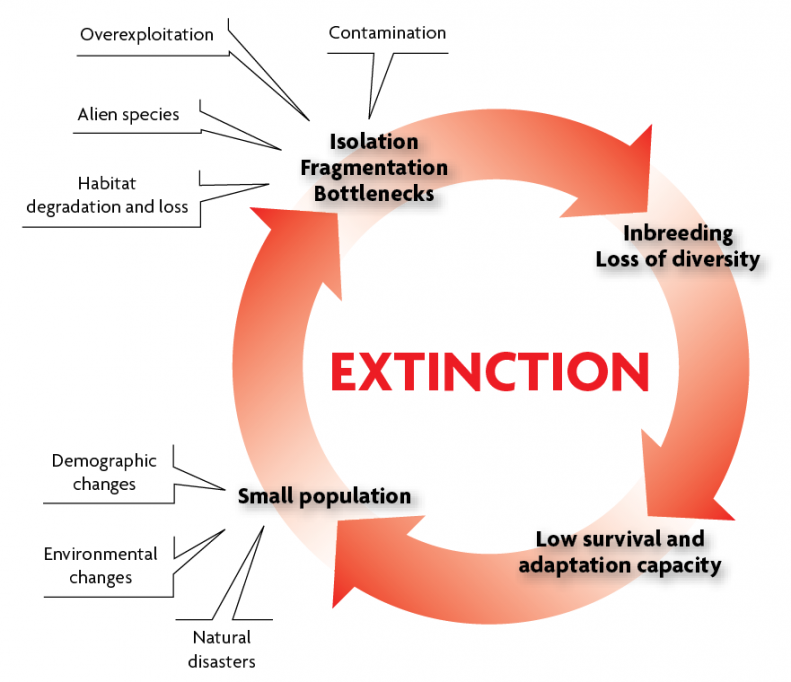
Extinction vortices
In practice, when a species is endangered and displays compromised average biological efficacy, it is difficult to discern to what extent this is due to genetic damage, and to evaluate the relative contribution of the accumulation of deleterious mutations, inbreeding depression and loss of genetic variability. In all likelihood it is a combination of these factors with other demographic, ecological, environmental or stochastic factors that together lead to extinction. This phenomenon is known as vortex or extinction vortex (Frankham et al., 2010), and is represented in Figure 2. Fluctuating environmental change, competition between species, the struggle for food resources, and natural disasters are among the natural causes of a decline in population size. Moreover, human action – embodied by habitat destruction and fragmentation, environmental degradation, overexploitation, and the introduction of alien species that displace native species – also leads to unremitting fragmentation and isolation of populations. As already highlighted, reduced population size generates greater losses of genetic variability through drift, as well as reductions in the reproductive capacity of individuals by inbreeding depression, and the accumulation of deleterious mutations. All these factors, in turn, contribute to a further decline in population size. The mutual reinforcement or synergy between demographic, genetic and stochastic effects will lead to extinction, the ultimate fate of small populations.
Conservation units
When designing a conservation programme, the first step is to decide which population should be maintained or what the conservation unit is. To do so, a number of ecological, cultural and economic considerations should be taken into account, as well as the extent to which the population is endangered and the likelihood of the programme succeeding (Oldenbroek, 1999). But it is also essential to define the genetic criteria by which we can determine priorities when it comes to maintaining or disposing of populations of the same species. The establishment of so-called «evolutionary significant units», which define populations to be given priority in conservation efforts, is a hotly debated topic at present because it is essential to define the conservation unit before starting an action programme (Allendorf et al., 2013). The use of genetic markers has become an essential tool for solving taxonomic problems and determining the genetic differences between groups. More recently, complementary methods have been incorporated that classify populations according to their ecological and genetic relationships. The grouping of populations following both criteria simultaneously can establish, with confidence, evolutionarily significant units that will be the basis of conservation programmes.

The cheetah is a species with low genetic variability due to bottlenecks that have occurred during its evolutionary history. / Ryan Taylor
Conservation programmes
From the genetic point of view, the goal of a conservation programme of a threatened or endangered species is to maintain the highest possible level of genetic variability, and to ensure the ability of individuals to survive, with the ultimate aim of facilitating their reintroduction in the wild. In conservation programmes in situ, namely conservation of populations in their own natural environment, basically the principles of action focus on taking the necessary measures for population size to increase. Among the various measures possible, we can mention the control or prohibition of hunting and fishing, the designation of nature reserves, the reduction of pollution and environmental degradation, the eradication of predators and competitors, the breakdown of barriers between isolated populations, and so on. Genetic aspects should also be taken into account for the proper implementation of these actions. For example, relocating individuals to another location in order to alleviate the negative effects of inbreeding should be done following genetic advice. Crossing genetically distant individuals (which usually correlates with physical distance) in an endeavour to improve hybrid vigour, can have the opposite effect, i.e., lower reproductive capacity of the offspring. One example of the problems caused by genetic mismanagement occurred when salmon rivers in the Cantabrian region of Spain were restocked with Scottish and Norwegian salmon from the 1970s to the 1990s. The poor adaptation of these alien fish to Spanish rivers was the main reason why the vast majority of the reintroduced fish did not reproduce successfully.

Programmes restocking Cantabrian salmon rivers with Scottish and Norwegian salmon in the seventies and nineties were unsuccessful due to the inability of the fish to adapt to the climatic conditions of the Spanish rivers. / Agencia SINC / Mine Obskuriteter
In ex situ conservation programmes, the population remains in captivity, either indefinitely or with the ultimate goal of reintroducing the conserved species in their natural habitat. It has been estimated that counting terrestrial vertebrates alone, some 2,000 species require captive breeding to save them from extinction (Frankham et al., 2010). These captive species are housed in zoos, wildlife parks, aquariums, botanical gardens and arboreta, as well as in germplasm banks, or via cryopreservation of plants and animals, cell cultures and gametes. A well known example of an endangered Iberian species subject to an important conservation programme is the Iberian lynx (Vargas 2009). The main objectives of ex situ conservation programmes are (Toro et al., 2009): first, avoid inbreeding (except in self-pollinating species), since increased inbreeding diminishes species viability; second, maintain the highest possible genetic variation, to safeguard the population by making it more resilient to new environmental challenges, and, finally, to protect the population from adapting to captivity, which would prejudice its success if reintroduced to the wild.

The Iberian lynx is one of Spain’s most representative endangered species, and an ambitious recovery programme is being run for its conservation. / CSIC
Since genetic resources are generally maintained in small population sizes, both in the case of animals in captivity and plants in germplasm banks, genetic drift is the main source of loss of genetic variation. Therefore the main principle to follow is to maintain the maximum effective size of the populations held in captivity, which can be achieved by the following guidelines below. First, increasing population size to the maximum possible, according to availability, and then maintain this size with the fewest temporary fluctuations possible; second, avoid mating between relatives; third, avoid bias in the sex ratio and minimize variation in parents’ contributions to offspring; and finally, through controlled migration, form connections between the reproductive nuclei that are kept apart due to logistic or natural reasons. Currently there are computational algorithms and optimization procedures that maintain maximum genetic diversity in captive populations if matings are controlled to some extent. These methods are becoming more sophisticated and incorporate, where possible, information on high-density genetic markers, obtained with new DNA sequencing technologies.
Future perspectives
The conservation of biodiversity is one of the most pressing problems caused by human population growth and irresponsible use of natural resources. Genetics and particularly population genetics, under development since the early twentieth century, answers many of the questions raised in situ regarding endangered species recovery and captive species management. The last two decades have witnessed a more dynamic application of genetic concepts to the conservation of biological diversity, making it one of the most active research fields. The management of ex situ conservation programmes will improve in the future thanks to the development of new reproductive technologies and cryopreservation, combined with ongoing advances in molecular techniques required for monitoring genetic diversity, and the development of approaches able to efficiently incorporate and collate all this information and make it available to conservation workers. Regarding in situ conservation programmes, the key may lie in improving the coordination of information from various sources (genetic, ecological, demographic, environmental), by developing global computing platforms that can combine a lot of information about individuals and populations at risk, and the habitats in which they live. However, genetic factors account for only one component of extinction risk. Fundamental threats reside in habitat loss, environmental degradation, global warming and overfishing, among other factors. It is in these crucial areas that governments should work more actively to curb the loss of biodiversity.
References
Allendorf, F. W.; Luikart, G. and S. N. Aitken, 2013. Conservation and the Genetics of Populations. 2nd edition. Wiley-Blackwell Publishing. Malden, USA.
Benito, C. and F. J. Espino, 2012. Genética, conceptos esenciales. Editorial Médica Panamericana. Madrid.
Frankham, R.; Ballou, J. D. and D. A. Briscoe, 2010. Introduction to Conservation Genetics. 2nd edition. Cambridge University Press. Cambridge.
Menotti-Raymond M. and S. J. O’Brien, 1995. «Evolutionary Conservation of Ten Microsatellite Loci in Four Species of Felidae». Journal of Heredity, 86(4): 319-322.
Oldenbroek, J. K., 1999. Genebanks and the Conservation of Farm Animal Genetic Resources. DLO Institute for Animal Sciences and Health. Lelystad, the Netherlands.
Toro, M. A., Fernández J. and A. Caballero, 2009. «Molecular Characterization of Breeds and Its Use in Conservation». Livestock Science, 120: 174-195. DOI: <10.1016/j.livsci.2008.07.003>.
Vargas, A., 2009. Conservación Ex-situ del Lince Ibérico: Un Enfoque Multidisciplinar. Fundación Biodiversidad. Madrid. Available at: <http://www.lynxexsitu.es/ficheros/documentos_pdf/37/bookexsituvargas2009_3.pdf>.