Hideous progeny?
The future of growing humans

Today’s biotechnologies are not simply providing powerful new possibilities in medicine; they are transforming our view of what it can mean to be human. In particular, the discovery of the extreme plasticity of cells – the possibility of changing one tissue type for another, and of regenerating the embryonic cell state from which we all grew – forces us to confront our status as a contingent community of living cells, and challenges traditional notions of self and identity. Here I discuss some of these technologies and their broader social, ethical and philosophical implications.
Keywords: tissue engineering, stem cells, organoids, cell reprogramming, transhumanism.
Visions of a full-grown human body assembled from component parts, from Mary Shelley’s Frankenstein (1818) to Karel Čapek’s 1921 play R.U.R. that introduced the word robot, remain fixated on the old Cartesian picture of body as mechanism. But if there is to be a future of «artificial» beings of flesh and blood, they will be grown, not built – recapitulating at least some of the process by which a ball of stem cells grows into a fetus in utero. As techniques for manipulating living cells become better understood, it has become possible to imagine creating humans from hand-crafted artificial aggregates of cells and tissues.
At what point, though, does an intervention in the process of human development make it artificial? Even before IVF, we spoke of artificial insemination, while IVF itself was insistently technologized through the label test-tube babies (test-tubes, a symbol of chemical synthesis, were never involved). Today’s controversial «artificial» interventions include embryo screening and selection, genome editing, and the allegedly «three-parent babies» of mitochondrial transfer. In some ways, the potential reproductive technologies described in this article are just the next step; it may well be that people whose developmental process started with one of these techniques will one day be deemed every bit as «normal» as people conceived by IVF are today. And perhaps that will be all to the good – but it does not mean that the ethical and social questions raised by these technologies are any less urgent.
Cell reprogramming
In 2006, Japanese biologist Shinya Yamanaka at Kyoto University and his coworkers found that mature, differentiated cells (an adult skin cell, say) can be turned into a stem-cell-like state by adding to it just four genes that are highly expressed in embryonic stem cells, for example using a virus as the gene-carrying vector – first for mice cells (Takahashi & Yamanaka, 2006), then human (Takahashi et al., 2007). Such reprogrammed cells are called induced pluripotent stem cells (iPSCs), and they can in principle be grown into any tissue type in the body by guiding the course of their further development. The discovery was not just a potentially valuable new tool for tissue engineering; it also overturned received wisdom by showing that cell «fates» are not fixed, and the developmental process of differentiation that produces them is not a one-way street. Cells are much more plastic and versatile than had been believed. It is now known that one mature cell type can also be directly transformed by similar means into another type without first reverting to a stem-cell state.
«At what point does an intervention in the process of human development make it artificial?»
When grown in culture in vitro, such reprogrammed cells may organize themselves into miniature, approximate versions of the respective structures and tissues they would adopt in an embryo. Kidney cells might develop into tiny kidney-like structures; gut cells start to form the tube-like, ciliated tissues of a gut; and neurons will take on some of the forms of the brain, such as the layers of a pseudo-cortex or the buds of a neural tube (Kim et al., 2020). These structures can serve as model systems for investigating development, as well as substrates for drug testing. It is hoped that they might also act as replacement parts that can be grafted into the body. If grown from iPSCs of the recipient, such transplants would incur none of the problems of immune rejection that plague organ transplants today.

In 2006, Japanese biologist Shinya Yamanaka at Kyoto University and his coworkers found that mature, differentiated cells (an adult skin cell, say) can be turned into a stem-cell-like state. Such reprogrammed cells are called induced pluripotent stem cells (iPSCs), and they can in principle be grown into any tissue type in the body by guiding the course of their further development. / National Institutes of Health
It is also possible to grow these «organoid» structures from normal embryonic stem cells (ESCs) taken from discarded IVF embryos. Both iPSCs and ESCs are at the core of modern techniques for cell transformation and culture, which already shows promise for, say, repairing damaged spinal cord (Nagoshi et al., 2019), reversing deterioration of vision (Li et al., 2017) and hearing (Tang et al., 2020), and restoring neurons in the brain lost to neurodegenerative diseases (Payne et al., 2015).
But the possibilities go way beyond regenerative medicine. These methods might create new possibilities for growing humans. In 2009 Kristin Baldwin of the Scripps Research Institute in California and her coworkers made full-grown mice from the skin cells (fibroblasts) of other mice (Boland et al., 2009). They reprogrammed the cells using the Yamanaka factors, and then injected these iPSCs into a mouse blastocyst embryo (a stage early in embryo growth when structure starts to appear) that had been manipulated to prevent its existing cells from developing further. The mouse fetus that developed from this blastocyst was then derived just from the iPSCs. Each of these fetuses grew to full-term pups that were delivered by caesarean section; about half of them survived and grew into adult mice with no apparent abnormalities. In other words, at least some iPSCs have the capacity to become whole new organisms. There is no obvious reason why this approach would not work with human cells, although at this stage such an experiment – with unknown health risks – would be deeply unethical and, in many countries, illegal.
Artificially constructed embryos (Simunovic & Brivanlou, 2017) are generally made from ESCs rather than iPSCs. Although ESCs taken from the inner cell mass of a blastocyst are able to grow in principle into any tissue type in the body, they cannot grow into a full embryo on their own because they have lost the ability to make the placenta and yolk sac – this is what distinguishes their «pluripotency» from the «totipotency» of the pre-blastocyst embryonic cells.
However, it has been known for over a decade that ESCs alone can nonetheless become somewhat embryo-like. In a culture medium, small clusters will spontaneously differentiate to form the three-layer structure that precedes gastrulation: the ectoderm (the progenitor of skin), mesoderm (blood, heart, kidneys, muscle, and other tissues) and endoderm (gut). There, however, the process typically stops, with these «embryoid bodies» as simple balls of cells with concentric layering. In a normally developing human embryo, this triple layer of cell types then begins to fold and take on the shape of the gastrula – the first appearance of a genuine body plan. But for that to happen, the embryo needs to be implanted in the uterus wall, a process that can be crudely mimicked using a biopolymer like collagen as the uterine proxy. One might get further along the developmental trajectory by adding the other tissues that embryos require. The simplest recipe involves just two types of cell: pluripotent ESCs and the cells that give rise to placenta, called trophoblast cells. The latter deliver signals to ESCs in utero which induce them to take on the shape of a gastrulated embryo.
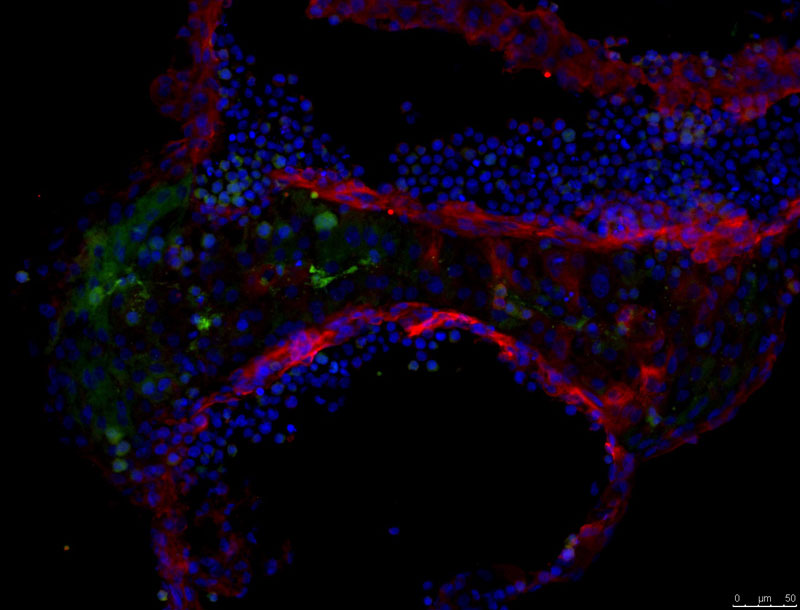
A brain organoid grown from the author’s skin cells, after reprogramming them as induced pluripotent stem cells. In this cross-section, different cell types are stained different colours, and some of the organizational features of brain tissue can be seen. / Image courtesy of Magdalena Zernicka-Goetz and Marta Shahbazi, University of Cambridge
In 2017 Magdalena Zernicka-Goetz and her colleagues in Cambridge used an extended recipe to create a more advanced form of mouse embryoid (Harrison et al., 2017). The two-component mixture still lacks another of the extra-embryonic cell types present in a normal embryo, called primitive endoderm cells. These cells form the yolk sac of the in utero embryo and supply signalling molecules needed to trigger the formation of the central nervous system. In this experiment the gel used as the culture medium could act as a crude substitute for the primitive endoderm: a scaffold that would hold the embryoid in place as the trophoblasts did their job. The composite structure developed the hollow shape of a gastrulated mouse embryo, the central void mimicking the amniotic cavity that forms in a normal embryo. The embryoid was now a «gastruloid».
Many of the basic processes in early mouse embryogenesis are the same as those in humans, but development becomes very different even at the stage of gastrulation: the mouse gastrula looks quite unlike the human one. Yet it is not obvious that there should be any fundamental obstacle to making human embryoids of at least this level of complexity. Human trophoblast cells – the vital ingredient for getting a placenta-like signal – can now be made from stem cells (Kojima et al., 2017). What’s more, human trophoblasts have been grown into organoids that mimic the placenta, raising the possibility of an in vitro tissue that can nurture embryoids as a stand-in for the maternal environment. Stem-cell biologist Martin Pera of the Jackson Laboratory in Maine (USA) says that «there is no reason to believe that there are any insurmountable barriers to the creation of cell culture entities that resemble the human post-implantation embryo in vitro» (Pera, 2017, p. 138).
New kinds of life
Perhaps the barriers are not technical but conceptual. We must ask: what sort of being is this? Embryoids and gastruloids are not exactly synthetic versions of the equivalent structures in normal embryogenesis, and none yet has the slightest potential to continue its growth in vitro towards a baby animal. They are a class of living things in their own right. Anticipating that the rudimentary embryoid structures so far created with human cells will evolve towards the more advanced forms made from mouse cells, George Church of Harvard University has proposed to call this family of existing and prospective living objects synthetic human entities with embryo-like features, or SHEEFs (Aach et al., 2017).
Most researchers in the field recommend a ban on the use of embryo-like entities made from stem cells for reproductive purposes. But even if this were not to mean «growing a human», research on ordinary human embryos is much more tightly constrained than that – many countries currently impose a 14-day limit on in vitro embryo growth. This is the stage at which normal human embryos develop the «primitive streak» that will eventually become the central nervous system: a crude developmental proxy of «personhood». But embryoids and SHEEFs might not follow this natural developmental pathway at all. If not, how can we decide what the limit should be for their development?
Partly for that reason, there is no consensus on how to legislate research on embryoids and SHEEFs. It is not just that their moral status is ambiguous; there is no standard form for an embryoid; they are put together however we want them, and the cells work with what they are given.
Rewriting the rules
Since the 1970s it has become possible to edit genomes: to excise or insert genes at will, sometimes from different species entirely. A technique called CRISPR, developed in 2012 largely by biochemists Emmanuelle Charpentier, Jennifer Doudna and Feng Zhang, has transformed the field because of the accuracy with which it can target and edit the genome (Adli, 2018). CRISPR exploits a family of natural DNA-cleaving enzymes in bacteria called Cas proteins – usually one denoted Cas9, but others find specialized uses too – to target and edit genes. The targeted section of DNA is recognized by a «guide RNA» molecule carried alongside Cas9.
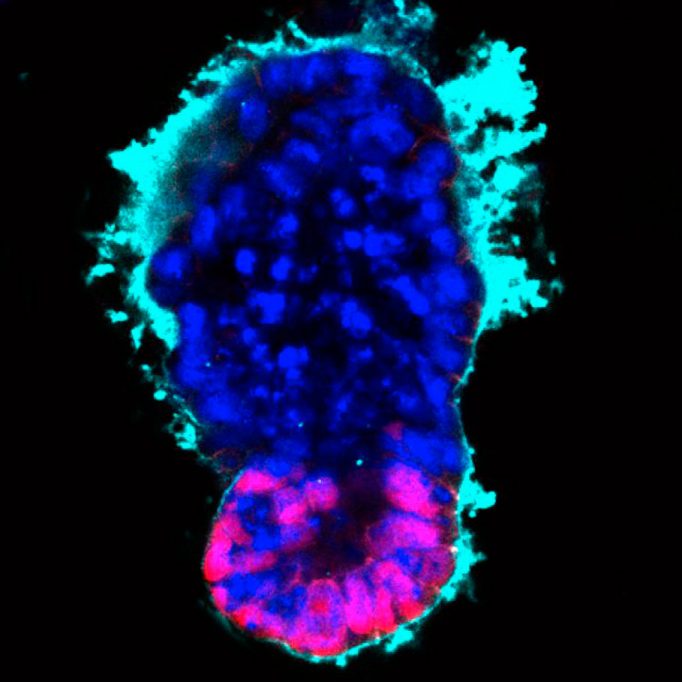
A «synthetic gastrula» or gastruloid made by Madgalena Zernicka-Goetz and colleagues by assembling embryonic stem cells (magenta) and pre-placental trophoblast (blue) cells within a synthetic «extracellular matrix» (cyan) that mimics the missing primitive endoderm cells – the critical third component of a true gastrulating embryo. / Image courtesy of Magdalena Zernicka-Goetz and Marta Shahbazi, University of Cambridge
CRISPR is more accurate, as well as cheaper, than previous gene-editing techniques. The method could potentially supply a powerful way to cure diseases caused by mutations of one or a few specific genes, such as muscular dystrophy and thalassemia. Human clinical trials of such treatments are now underway.
These candidate gene therapies aim to alter genes in somatic cells. Any changes to genes made in an early embryo, however, will be incorporated into the germline and passed down future generations. For that reason, scientists are hesitant about introducing changes to the germline. What is more, if the editing process makes any other inadvertent alterations to the genome at this early stage in development, those changes will be spread throughout the body as the embryo grows.
CRISPR has already been used to genetically modify human embryos, purely to see if it is possible in principle. But the use of CRISPR for human reproduction is forbidden in all countries that legislate on it, and is almost wholly rejected in principle by the medical research community. However, they were shocked and dismayed when in late 2018 Chinese biologist He Jiankui announced that he had used the method to modify several IVF embryos and implanted them in several women, one of whom had already given birth to twins. He used CRISPR to alter a gene called CCR5, which is involved in infection of cells by the AIDS virus HIV, so as to hinder the virus from entering cells – conflicting with the general view that no gene editing should be considered unless it addressed an unmet medical need. To make matters worse, the work seemed to have been done shoddily, with some off-target genome modification.
«Genes do not work in the way that would be needed for reliable, predictive production of “designer babies”»
Yet there is no obvious reason why genome editing for human reproduction should be forever ruled out (Greely, 2021). In those relatively rare cases where a debilitating disease is caused by a single gene, and the consequences of replacing a faulty with a healthy version can be reliably predicted, there may be a place for it eventually in reproductive medicine. Eliminating such diseases from the germline seems an unqualified good: not only the person grown from the modified embryo but also their offspring too would be free of the disease.
What, then, is to stop us now from altering the genes of IVF embryos to select or enhance the traits of the resulting child? Might we tailor her (for we can certainly select the sex) to have flame-red hair and green eyes, to be smart and athletic, full of grace and musical ability – a stereotypical wish-list for «designer babies»?
Such discussions are, however, often genetically naïve. There is rarely a straightforward one-to-one relationship between genes and traits, especially those that might be likely targets of «positive selection» (whether by gene-editing or selection of IVF embryos using the existing technique of preimplantation genetic diagnosis, PGD). The genetic basis of attributes like intelligence and musicality is too thinly spread throughout the genome to make them significantly amenable to gene editing (even eye colour, long thought to involve just a few genes, turns out to have a more complicated genetic basis). One would need to edit hundreds, perhaps thousands of genes – which would not only be impractical but would also surely have unpredictable consequences for other traits. Genes do not work in the way that would be needed for reliable, predictive production of «designer babies» that are smarter, stronger, or more attractive.
At any rate, if there is going to be anything even vaguely resembling the popular designer-baby fantasy – genetic selection for non-medical reasons – it will come first from embryo selection through PGD, not gene editing. «Almost everything you can accomplish by gene editing, you can accomplish by embryo selection», says bioethicist Hank Greely of Stanford University.
If PGD were to become routine and liberalized (some countries already permit it for selecting the sex of a child), the ethical issues are complex (Greely, 2016). The siren allure of selecting the «perfect child» via PGD could drive expectations to pathological extremes. What if the child genetically selected in the hope that they will have superior athletic ability or artistic talent fails to deliver – as some inevitably will, given that such predictions are only probabilistic? And unequal availability of choice to different socioeconomic sectors of the population could seriously disturb social stability, leading to a social or even a national «genetic divide» of the kind portrayed in the 1997 movie Gattaca.
Artificial gametes
Given the current expense, uncertainty and grueling nature of IVF, PGD seems unlikely to become a reproductive method of choice in the near term. But cell reprogramming could change that too. One of the key limitations to current IVF is the difficulty of obtaining eggs for fertilization. Any given round of egg extraction typically produces half a dozen or so that can be used, and not all of these will become viable embryos for implantation.

The research community was shocked and dismayed when in late 2018 Chinese biologist He Jiankui announced that he had used the CRISPR method, resulting in the birth of twin girls with edited genomes to avoid an HIV infection. In 2020, the scientist was suspended and sentenced to three years in prison. In the picture, He Jiankui explains the results of the experiment in a YouTube video published in November 2018 in the channel The He Lab. / The He Lab
But cell-transformation technologies could permit the manufacture of eggs in vitro by reprogramming somatic cells into gametes. This is harder than most other cell types, because gametes (eggs and sperm) have only one copy of the chromosomes, while other cells have two. They are made not by regular cell division (mitosis) but by a special process called meiosis, which halves their number of chromosomes.
It is not at all easy to recapitulate that process in a petri dish. But making fully functional eggs from iPSCs has already been achieved in mice by Mitinori Saitou and coworkers in Kyoto (Saitou & Miyauchi, 2016). First they transformed iPSCs in vitro into precursors of gametes, called primordial germ cells (PGCs), which have not yet undergone meiosis and cannot be fertilized. Then they completed the maturation process in vivo by transplanting the PGCs into the ovaries of live mice. «Artificial» sperm has also been produced this way by transplanting PGCs made from iPSCs into the testes of adult mice. If it works in humans, this could offer a remedy for low sperm production, a common cause of fertility problems.
Beyond flesh
The possibilities for growing and shaping human beings provided by our new technologies for manipulating cells might seem dramatic, even alarming, but they are rather conservative compared to what some of scientists in the early days of the field foresaw. In his 1929 essay The World, The Flesh and the Devil, on the potential of future biotechnologies, J. Desmond Bernal (1970) speculated and extrapolated far beyond what many scientists would be willing to risk today. We might, he said, eventually get rid of «the useless parts of the body» and replace them with mechanical devices: artificial limbs and sensory devices that do a much better job, until we reach the stage of a living brain connected to and operating a machine-like «body».
Bernal’s speculations are now regarded as a part of the intellectual heritage of the movement called transhumanism, which seeks to use technologies to extend the possibilities of the human body in radical ways. According to Max More, CEO of the cryonics company Alcor Life Extension Foundation, transhumanism «seek[s] the continuation and acceleration of the evolution of intelligent life beyond its currently human form and the human limitations by means of science and technology, guided by life-promoting principles and values» (More & Vita-More, 2013, p.3).
Much of the transhumanist programme so far has focused on the extension of cognitive and sensory capabilities using medical and information technologies, drugs, and human-machine interfaces. The plasticity of human flesh itself is now poised to become another tool in the transhumanists’ toolkit.
«Transhumanism is challenging our notions of self and identity»
To what end? Transhumanists assert the right of individuals to reimagine and reconfigure their own bodies and minds, including the right to extend lifespan and augment physical and mental capabilities. Mostly they exhibit a libertarian tendency, emphasizing the rights of the individual and casting the possibilities in a utopian light. Yet most of our narratives about such efforts tend towards the dystopian. One danger of transhumanism is not that it poses hubristic questions and challenges – today’s biotechnologies do that already – but that it is too readily appropriated by false prophets and technological fantasists in pursuit of their own obsessions.
But the movement motivates some serious ethical reflection. We already put a great deal of effort into seeking what we consider to be the «good life»: extending the period of good bodily and mental health, cultivating meaningful relationships, alleviating suffering in others, respecting individual autonomy and rights, deepening our intellectual and emotional engagement with the world. If the technologies of medicine and information can afford new opportunities towards these goals, why would it be anything but ethically wise and responsible to take them?
The principle of redesigning the body to extend its capabilities is nothing more than we have practised for centuries, at least since the development of prostheses and aids for vision and hearing. We can now create artificial limbs that respond to nerve impulses or eye-tracking screens, and on-skin or internally implanted radiofrequency devices that can monitor and broadcast physiological indicators of health. Technologies of cell transformation might soon render possible much more remarkable morphological changes of our bodies, and the transhumanist perspective can be a useful and even essential part of the debate about what is possible and desirable. If we are to seriously engage with these ideas, we must recognize that the issues reach even beyond the social and ethical to the philosophical, challenging our notions of self and identity. They force us to reconsider what it means to be human, or in the words of the American cultural theorist Susan Merrill Squier (2004), to «replot the human».
References
Aach, J., Lunshof, J., Iyer, E., & Church, G. M. (2017). Addressing the ethical issues raised by synthetic human entities with embryo-like features. eLife, 6, e20674. https://doi.org/10.7554/eLife.20674
Adli, M. (2018). The CRISPR tool kit for genome editing and beyond. Nature Communications, 9, 1911. https://doi.org/10.1038/s41467-018-04252-2
Bernal, J. D. (1970). The world, the flesh and the devil: An enquiry into the future of the three enemies of the rational soul. Jonathan Cape. (Original work published in 1931).
Boland, M. J., Hazen, J. L., Nazor, K. L., Rodriguez, A. R., Gifford, W., Martin, G., Kupriyanov, S., & Baldwin, K. K. (2009). Adult mice generated from induced pluripotent stem cells. Nature, 461, 91–94. https://doi.org/10.1038/nature08310
Greely, H. T. (2016). The end of sex and the future of human reproduction. Harvard University Press.
Greely, H. T. (2021). CRISPR people: The science and ethics of editing humans. MIT Press.
Harrison, S. E., Sozen, B., Christodoulou, N., Kyprianou, C., & Zernicka-Goetz, M. (2017). Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science, 356(6334), eaal1810. https://doi.org/10.1126/science.aal1810
Kim, J., Koo, B. K., & Knoblich, J. A. (2020). Human organoids: Model systems for human biology and medicine. Nature Reviews Molecular Cell Biology, 21, 571–584. https://doi.org/10.1038/s41580-020-0259-3
Kojima, J., Fukuda, A., Taira, H., Kawasaki, T., Ito, H., Kuji, N., Isaka, K., Umezawa, A., & Akutsu, H. (2017). Efficient production of trophoblast lineage cells from human induced pluripotent stem cells. Laboratory Investigation, 97, 1188–1200. https://doi.org/10.1038/labinvest.2016.159
Li, F., Hu, J., & He, T.-C. (2017). iPSC-based treatment of age-related macular degeneration (AMD): The path to success requires more than blind faith. Genes & Disease, 4(2), 41–42. https://doi.org/10.1016/j.gendis.2017.03.001
More, M., & Vita-More, N. (Eds.). (2013). The transhumanist reader. Wiley-Blackwell.
Nagoshi, N., Tsuji, O., Nakamura, M., & Okano, H. (2019). Cell therapy for spinal cord injury using induced pluripotent stem cells. Regenerative Therapy, 11, 75–80. https://doi.org/10.1016/j.reth.2019.05.006
Payne, N. L., Sylvain, A., O’Brien, C., Herszfeld, D., Sun, G., & Bernard, C. C. A. (2015). Application of human induced pluripotent stem cells for modeling and treating neurodegenerative diseases. New Biotechnology, 32(1), 212–228. https://doi.org/10.1016/j.nbt.2014.05.001
Pera, M. (2017). Embryogenesis in a dish. Science, 356(6334), 137–138. https://doi.org/10.1126/science.aan1495
Saitou, M., & Miyauchi, H. (2016). Gametogenesis from pluripotent stem cells. Cell Stem Cell, 18(6), 721–735. https://doi.org/10.1016/j.stem.2016.05.001
Simunovic, M., & Brivanlou, A. H. (2017). Embryoids, organoids and gastruloids: New approaches to understanding embryogenesis. Development, 144(6), 976–985. https://doi.org/10.1242/dev.143529
Squier, S. M. (2004). Liminal lives: Imagining the human at the frontiers of biomedicine. Duke University Press. https://doi.org/10.1215/9780822386285
Tang, P. C., Hashino, E., & Nelson, R. F. (2020). Progress in modeling and targeting inner ear disorders with pluripotent stem cells. Stem Cell Reports, 14(6), 996–1008. https://doi.org/10.1016/j.stemcr.2020.04.008
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomodad, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872. https://doi.org/10.1016/j.cell.2007.11.019
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676. https://doi.org/10.1016/j.cell.2006.07.024